Improving the Specificity of Screening for Lethal Prostate Cancer Using Prostate-specific Antigen and a Panel of Kallikrein Markers: A Nested Case-Control Study
- PMID: 25682340
- PMCID: PMC4496315
- DOI: 10.1016/j.eururo.2015.01.009
Improving the Specificity of Screening for Lethal Prostate Cancer Using Prostate-specific Antigen and a Panel of Kallikrein Markers: A Nested Case-Control Study
Abstract
Background: A disadvantage of prostate-specific antigen (PSA) for the early detection of prostate cancer (PCa) is that many men must be screened, biopsied, and diagnosed to prevent one death.
Objective: To increase the specificity of screening for lethal PCa at an early stage.
Design, setting, and participants: We conducted a case-control study nested within a population-based cohort. PSA and three additional kallikreins were measured in cryopreserved blood from a population-based cohort in Västerbotten, Sweden. Of 40379 men providing blood at ages 40, 50, and 60 yr from 1986 to 2009, 12542 men were followed for >15 yr. From this cohort, the Swedish Cancer Registry identified 1423 incident PCa cases, 235 with distant metastasis.
Outcome measurements and statistical analysis: Risk of distant metastasis for different PSA levels and a prespecified statistical model based on the four kallikrein markers.
Results and limitations: Most metastatic cases occurred in men with PSA in the top quartile at age 50 yr (69%) or 60 yr (74%), whereas 20-yr risk of metastasis for men with PSA below median was low (≤0.6%). Among men with PSA >2 ng/ml, a prespecified model based on four kallikrein markers significantly enhanced the prediction of metastasis compared with PSA alone. About half of all men with PSA >2 ng/ml were defined as low risk by this model and had a ≤1% 15-yr risk of metastasis.
Conclusions: Screening at ages 50-60 yr should focus on men with PSA in the top quartile. A marker panel can aid biopsy decision making.
Patient summary: For men in their fifties, screening should focus on those in the top 10% to 25% of PSA values because the majority of subsequent cases of distant metastasis are found among these men. Testing of four kallikrein markers in men with an elevated PSA could aid biopsy decision making.
Keywords: Cancer metastasis; Kallikrein-related peptidases; Prostate biopsy; Prostate cancer; Prostate-specific antigen.
Copyright © 2015 European Association of Urology. Published by Elsevier B.V. All rights reserved.
Figures
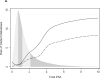

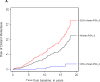

Comment in
-
The Era of Prostate-specific Antigen-based Personalized Prostate Cancer Screening Has Only Just Begun.Eur Urol. 2015 Aug;68(2):214-5. doi: 10.1016/j.eururo.2015.01.031. Epub 2015 Feb 11. Eur Urol. 2015. PMID: 25680240 No abstract available.
-
Re: Improving the Specificity of Screening for Lethal Prostate Cancer Using Prostate-Specific Antigen and a Panel of Kallikrein Markers: A Nested Case-Control Study.J Urol. 2015 Aug;194(2):393-4. doi: 10.1016/j.juro.2015.05.066. Epub 2015 May 18. J Urol. 2015. PMID: 26195364 No abstract available.
Similar articles
-
Twenty-year Risk of Prostate Cancer Death by Midlife Prostate-specific Antigen and a Panel of Four Kallikrein Markers in a Large Population-based Cohort of Healthy Men.Eur Urol. 2018 Jun;73(6):941-948. doi: 10.1016/j.eururo.2018.02.016. Epub 2018 Mar 5. Eur Urol. 2018. PMID: 29519548 Free PMC article.
-
Predictive value of four kallikrein markers for pathologically insignificant compared with aggressive prostate cancer in radical prostatectomy specimens: results from the European Randomized Study of Screening for Prostate Cancer section Rotterdam.Eur Urol. 2013 Nov;64(5):693-9. doi: 10.1016/j.eururo.2013.04.040. Epub 2013 May 2. Eur Urol. 2013. PMID: 23683475 Free PMC article. Clinical Trial.
-
A "PSA pyramid" for men with initial prostate-specific antigen ≤3 ng/ml: a plea for individualized prostate cancer screening.Eur Urol. 2015 Oct;68(4):591-7. doi: 10.1016/j.eururo.2014.04.005. Epub 2014 Apr 18. Eur Urol. 2015. PMID: 24794075 Clinical Trial.
-
4-Kallikrein Test and Kallikrein Markers in Prostate Cancer Screening.Urol Clin North Am. 2016 Feb;43(1):39-46. doi: 10.1016/j.ucl.2015.08.004. Urol Clin North Am. 2016. PMID: 26614027 Review.
-
[PSA and blood test diagnostics of prostate cancer].Duodecim. 2015;131(17):1547-52. Duodecim. 2015. PMID: 26548101 Review. Finnish.
Cited by
-
PRECISION MANAGEMENT OF LOCALIZED PROSTATE CANCER.Expert Rev Precis Med Drug Dev. 2016;1(6):505-515. doi: 10.1080/23808993.2016.1267562. Epub 2016 Dec 12. Expert Rev Precis Med Drug Dev. 2016. PMID: 28133630 Free PMC article.
-
Clinical performance of the 4Kscore Test to predict high-grade prostate cancer at biopsy: A meta-analysis of us and European clinical validation study results.Rev Urol. 2017;19(3):149-155. doi: 10.3909/riu0776. Rev Urol. 2017. PMID: 29302237 Free PMC article.
-
Biomarkers in localized prostate cancer.Future Oncol. 2016 Feb;12(3):399-411. doi: 10.2217/fon.15.318. Epub 2016 Jan 15. Future Oncol. 2016. PMID: 26768791 Free PMC article. Review.
-
Proteomic analyses identify major vault protein as a prognostic biomarker for fatal prostate cancer.Carcinogenesis. 2021 May 28;42(5):685-693. doi: 10.1093/carcin/bgab015. Carcinogenesis. 2021. PMID: 33609362 Free PMC article.
-
Risk factors of developing visceral metastases at diagnosis in prostate cancer patients.Transl Cancer Res. 2019 Jun;8(3):928-938. doi: 10.21037/tcr.2019.05.31. Transl Cancer Res. 2019. PMID: 35116832 Free PMC article.
References
-
- Hallmans G, Agren A, Johansson G, et al. Cardiovascular disease and diabetes in the Northern Sweden Health and Disease Study Cohort--evaluation of risk factors and their interactions. Scand J Public Health Suppl. 2003;61:18–24. - PubMed
-
- Weinehall L, Hallgren CG, Westman G, Janlert U, Wall S. Reduction of selection bias in primary prevention of cardiovascular disease through involvement of primary health care. Scand J Prim Health Care. 1998;16:171–6. - PubMed
-
- Pilebro B, Johansson R, Damber L, Damber JE, Stattin P. Population-based study of prostate-specific antigen testing and prostate cancer detection in clinical practice in northern Sweden. Scand J Urol Nephrol. 2003;37:210–2. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

