Orthotopic non-metastatic and metastatic oral cancer mouse models
- PMID: 25682387
- PMCID: PMC4427342
- DOI: 10.1016/j.oraloncology.2015.01.012
Orthotopic non-metastatic and metastatic oral cancer mouse models
Abstract
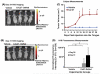
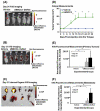
Oral cancer is characterized by high morbidity and mortality with a predisposition to metastasize to different tissues, including lung, liver, and bone. Despite progress in the understanding of mutational profiles and deregulated pathways in oral cancer, patient survival has not significantly improved over the past decades. Therefore, there is a need to establish in vivo models that recapitulate human oral cancer metastasis to evaluate therapeutic potential of novel drugs. Here we report orthotopic tongue cancer nude mouse models to study oral cancer growth and metastasis using human metastatic (UMSCC2) and non-metastatic (CAL27) cell lines, respectively. Transduction of these cell lines with lentivirus expressing red fluorescent protein (DsRed) followed by injection into tongues of immunodeficient mice generated orthotopic tongue tumors that could be monitored for growth and metastasis by fluorescence measurement with an in vivo Imaging System (IVIS 200). The growth rates of CAL27-DsRed induced tumors were higher than UMSCC2-DsRed tumors after day 15, while UMSCC2-DsRed tumors revealed metastasis beginning on day 21. Importantly, UMSCC2 tumors metastasized to a number of tissues including the submandibular gland, lung, kidney, liver, and bone. Further, immunohistochemical analyses of tongue tumors induced by CAL27 and UMSCC2 cells revealed elevated expression of components of protumorigenic pathways deregulated in human cancers, including Cyclin D1, PCNA, Ki-67, LSD1, LOXL2, MT-MMP1, DPAGT1, E-cadherin, OCT4A, and H3K4me1/2. These orthotopic mouse models are likely to be useful tools for gaining insights into the activity and mechanisms of novel oral cancer drug candidates.
Keywords: Mouse models; Oral cancer; Orthotopic tumors metastasis.
Copyright © 2015 Elsevier Ltd. All rights reserved.
Figures
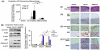

Similar articles
-
An orthotopic nude mouse model of oral tongue squamous cell carcinoma.Clin Cancer Res. 2002 Jan;8(1):293-8. Clin Cancer Res. 2002. PMID: 11801572
-
Development and optimization of orthotopic liver metastasis xenograft mouse models in uveal melanoma.J Transl Med. 2020 May 20;18(1):208. doi: 10.1186/s12967-020-02377-x. J Transl Med. 2020. PMID: 32434572 Free PMC article.
-
In Vivo Selection of Intermediately- and Highly-Malignant Variants of Triple-negative Breast Cancer in Orthotopic Nude Mouse Models.Anticancer Res. 2016 Dec;36(12):6273-6277. doi: 10.21873/anticanres.11222. Anticancer Res. 2016. PMID: 27919946
-
Animal models of bone metastasis.Cancer. 2003 Feb 1;97(3 Suppl):748-57. doi: 10.1002/cncr.11150. Cancer. 2003. PMID: 12548572 Review.
-
Orthotopic metastatic mouse models for anticancer drug discovery and evaluation: a bridge to the clinic.Invest New Drugs. 1999;17(4):343-59. doi: 10.1023/a:1006326203858. Invest New Drugs. 1999. PMID: 10759402 Review.
Cited by
-
Inhibition of LSD1 epigenetically attenuates oral cancer growth and metastasis.Oncotarget. 2017 Jul 27;8(43):73372-73386. doi: 10.18632/oncotarget.19637. eCollection 2017 Sep 26. Oncotarget. 2017. PMID: 29088714 Free PMC article.
-
Impact of Epigenetic Regulation on Head and Neck Squamous Cell Carcinoma.J Dent Res. 2019 Mar;98(3):268-276. doi: 10.1177/0022034518816947. Epub 2019 Jan 7. J Dent Res. 2019. PMID: 30615537 Free PMC article.
-
Targeting oral cancer epigenome via LSD1.Aging (Albany NY). 2017 Dec 11;9(12):2455-2456. doi: 10.18632/aging.101343. Aging (Albany NY). 2017. PMID: 29232656 Free PMC article. No abstract available.
-
Inactivation of homeodomain-interacting protein kinase 2 promotes oral squamous cell carcinoma metastasis through inhibition of P53-dependent E-cadherin expression.Cancer Sci. 2021 Jan;112(1):117-132. doi: 10.1111/cas.14691. Epub 2020 Nov 9. Cancer Sci. 2021. PMID: 33063904 Free PMC article.
-
Mouse Tumor-Bearing Models as Preclinical Study Platforms for Oral Squamous Cell Carcinoma.Front Oncol. 2020 Feb 25;10:212. doi: 10.3389/fonc.2020.00212. eCollection 2020. Front Oncol. 2020. PMID: 32158692 Free PMC article. Review.
References
-
- Choi S, Myers JN. Molecular pathogenesis of oral squamous cell carcinoma: implications for therapy. J Dent Res. 2008;87:14–32. - PubMed
-
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. - PubMed
-
- Marsh D, Suchak K, Moutasim KA, Vallath S, Hopper C, Jerjes W, et al. Stromal features are predictive of disease mortality in oral cancer patients. J Pathol. 2011;223:470–81. - PubMed
-
- Haddad RI, Shin DM. Recent advances in head and neck cancer. New Engl J Med. 2008;359:1143–54. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

