PD-1 pathway inhibitors: the next generation of immunotherapy for advanced melanoma
- PMID: 25682878
- PMCID: PMC4414130
- DOI: 10.18632/oncotarget.2980
PD-1 pathway inhibitors: the next generation of immunotherapy for advanced melanoma
Abstract
Checkpoint inhibitors are revolutionizing treatment options and expectations for patients with melanoma. Ipilimumab, a monoclonal antibody against cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4), was the first approved checkpoint inhibitor. Emerging long-term data indicate that approximately 20% of ipilimumab-treated patients achieve long-term survival. The first programmed death 1 (PD-1) inhibitor, pembrolizumab, was recently approved by the United States Food and Drug Administration for the treatment of melanoma; nivolumab was previously approved in Japan. PD-1 inhibitors are also poised to become standard of care treatment for other cancers, including non-small cell lung cancer, renal cell carcinoma and Hodgkin's lymphoma. Immunotherapy using checkpoint inhibition is a different treatment approach to chemotherapy and targeted agents: instead of directly acting on the tumor to induce tumor cell death, checkpoint inhibitors enhance or de novo stimulate antitumor immune responses to eliminate cancer cells. Initial data suggest that objective anti-tumor response rates may be higher with anti-PD-1 agents compared with ipilimumab and the safety profile may be more tolerable. This review explores the development and next steps for PD-1 pathway inhibitors, including discussion of their novel mechanism of action and clinical data to-date, with a focus on melanoma.
Conflict of interest statement
Dr. Luke reports consultancy and travel from Amgen, Bayer, and Genentech, and clinical trial support to his institution from EMD Serono, GlaxoSmithKline, and Novartis.
Dr. Ott reports consultancy from Bristol-Myers Squibb and clinical trial support to his institution from ARMO BioSciences, Bristol-Myers Squibb, MedImmune, and Merck.
Figures
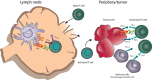

References
-
- Barth A, Wanek LA, Morton DL. Prognostic factors in 1,521 melanoma patients with distant metastases. J Am Coll Surg. 1995;181:193–201. - PubMed
-
- Manola J, Atkins M, Ibrahim J, Kirkwood J. Prognostic factors in metastatic melanoma: a pooled analysis of Eastern Cooperative Oncology Group trials. J Clin Oncol. 2000;18:3782–3793. - PubMed
-
- Unger JM, Flaherty LE, Liu PY, Albain KS, Sondak VK. Gender and other survival predictors in patients with metastatic melanoma on Southwest Oncology Group trials. Cancer. 2001;91:1148–1155. - PubMed
-
- Flaherty KT, Robert C, Hersey P, Nathan P, Garbe C, Milhem M, Demidov LV, Hassel JC, Rutkowski P, Mohr P, Dummer R, Trefzer U, Larkin JM, et al. Improved survival with MEK inhibition in BRAF-mutated melanoma. N Engl J Med. 2012;367:107–114. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

