Insulin degludec in combination with bolus insulin aspart is safe and effective in children and adolescents with type 1 diabetes
- PMID: 25683037
- PMCID: PMC4413367
- DOI: 10.1111/pedi.12263
Insulin degludec in combination with bolus insulin aspart is safe and effective in children and adolescents with type 1 diabetes
Abstract
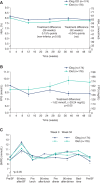
Insulin degludec (IDeg) once-daily was compared with insulin detemir (IDet) once- or twice-daily, with prandial insulin aspart in a treat-to-target, randomized controlled trial in children 1-17 yr with type 1 diabetes, for 26 wk (n = 350), followed by a 26-wk extension (n = 280). Participants were randomized to receive either IDeg once daily at the same time each day or IDet given once or twice daily according to local labeling. Aspart was titrated according to a sliding scale or in accordance with an insulin:carbohydrate ratio and a plasma glucose correction factor. Randomization was age-stratified: 85 subjects 1-5 yr. (IDeg: 43), 138 6-11 yr (IDeg: 70) and 127 12-17 yr (IDeg: 61) were included. Baseline characteristics were generally similar between groups overall and within each stratification. Non-inferiority of IDeg vs. IDet was confirmed for HbA1c at 26 wk; estimated treatment difference (ETD) 0.15% [-0.03; 0.32]95% CI . At 52 wk, HbA1c was 7.9% (IDeg) vs. 7.8% (IDet), NS; change in mean FPG was -1.29 mmol/L (IDeg) vs. +1.10 mmol/L (IDet) (ETD -1.62 mmol/L [-2.84; -0.41]95% CI , p = 0.0090) and mean basal insulin dose was 0.38 U/kg (IDeg) vs. 0.55 U/kg (IDet). The majority of IDet treated patients (64%) required twice-daily administration to achieve glycemic targets. Hypoglycemia rates did not differ significantly between IDeg and IDet, but confirmed and severe hypoglycemia rates were numerically higher with IDeg (57.7 vs. 54.1 patient-years of exposure (PYE) [NS] and 0.51 vs. 0.33, PYE [NS], respectively) although nocturnal hypoglycemia rates were numerically lower (6.0 vs. 7.6 PYE, NS). Rates of hyperglycemia with ketosis were significantly lower for IDeg vs. IDet [0.7 vs. 1.1 PYE, treatment ratio 0.41 (0.22; 0.78)95% CI , p = 0.0066]. Both treatments were well tolerated with comparable rates of adverse events. IDeg achieved equivalent long-term glycemic control, as measured by HbA1c with a significant FPG reduction at a 30% lower basal insulin dose when compared with IDet. Rates of hypoglycemia did not differ significantly between the two treatment groups; however, hyperglycemia with ketosis was significantly reduced in those treated with IDeg.
Trial registration: ClinicalTrials.gov NCT01513473.
Keywords: adolescents; children; insulin degludec; type 1 diabetes.
© 2015 The Authors. Pediatric Diabetes published by John Wiley & Sons Ltd.
Figures




References
-
- The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications of insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:979–986. - PubMed
-
- The Diabetes Control and Complications Trial Research Group. Adverse events and their association with treatment regimens in the Diabetes Control and Complications Trial. Diabetes Care. 1995;18:1415–1427. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

