Cole-Carpenter syndrome is caused by a heterozygous missense mutation in P4HB
- PMID: 25683117
- PMCID: PMC4375435
- DOI: 10.1016/j.ajhg.2014.12.027
Cole-Carpenter syndrome is caused by a heterozygous missense mutation in P4HB
Abstract
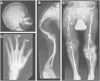
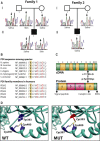
Cole-Carpenter syndrome is a severe bone fragility disorder that is characterized by frequent fractures, craniosynostosis, ocular proptosis, hydrocephalus, and distinctive facial features. To identify the cause of Cole-Carpenter syndrome in the two individuals whose clinical results were presented in the original description of this disorder, we performed whole-exome sequencing of genomic DNA samples from both individuals. The two unrelated individuals had the same heterozygous missense mutation in exon 9 of P4HB (NM_000918.3: c.1178A>G [p.Tyr393Cys]), the gene that encodes protein disulfide isomerase (PDI). In one individual, the P4HB mutation had arisen de novo, whereas in the other the mutation was transmitted from the clinically unaffected father who was a mosaic carrier of the variant. The mutation was located in the C-terminal disulfide isomerase domain of PDI, sterically close to the enzymatic center, and affected disulfide isomerase activity in vitro. Skin fibroblasts showed signs of increased endoplasmic reticulum stress, but despite the reported importance of PDI for collagen type I production, the rate of collagen type I secretion appeared normal. In conclusion, Cole-Carpenter syndrome is caused by a specific de novo mutation in P4HB that impairs the disulfide isomerase activity of PDI.
Copyright © 2015 The American Society of Human Genetics. Published by Elsevier Inc. All rights reserved.
Figures


References
-
- Cole D.E., Carpenter T.O. Bone fragility, craniosynostosis, ocular proptosis, hydrocephalus, and distinctive facial features: a newly recognized type of osteogenesis imperfecta. J. Pediatr. 1987;110:76–80. - PubMed
-
- Cohen M.M., Jr. Craniosynostosis update 1987. Am. J. Med. Genet. Suppl. 1988;4:99–148. - PubMed
-
- Amor D.J., Savarirayan R., Schneider A.S., Bankier A. New case of Cole-Carpenter syndrome. Am. J. Med. Genet. 2000;92:273–277. - PubMed
-
- Rauch F., Glorieux F.H. Osteogenesis imperfecta. Lancet. 2004;363:1377–1385. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

