Validation study of the combined repeated-dose toxicity and genotoxicity assay using gpt delta rats
- PMID: 25683344
- PMCID: PMC4452153
- DOI: 10.1111/cas.12634
Validation study of the combined repeated-dose toxicity and genotoxicity assay using gpt delta rats
Abstract
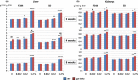
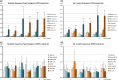
Transgenic rodents carrying reporter genes to detect organ-specific in vivo genetic alterations are useful for risk assessment of genotoxicity that causes cancer. Thus, the Organization for Economic Co-operation and Development has established the guideline for genotoxicity tests using transgenic animals, which may be combined with repeated-dose toxicity studies. Here, we provide evidence to support equivalence of gpt delta and wild type (WT) rats in terms of toxicological responses to a genotoxic hepatocarcinogen, N-nitrosodiethylamine (DEN), and a non-genotoxic hepatocarcinogen, di(2-ethylhexyl)phthalate (DEHP). gpt delta rats treated with DEHP showed similar increases in liver and kidney weights, serum albumin, albumin/globulin ratios, and incidence of diffuse hepatocyte hypertrophy compared to WT F344 and Sprague-Dawley (SD) rats. DEN-treated gpt delta rats showed equivalent increases in the number and area of precancerous GST-P-positive foci in the liver compared to WT rats. The livers of DEN-treated gpt delta rats also showed increased frequencies of gpt and Spi(-) mutations; such changes were not observed in DEHP-treated gpt delta rats. These results indicated that gpt delta rats (both F344 and SD backgrounds) showed comparable DEHP-induced toxicity and DEN-induced genotoxicity to those observed in WT rats. With regard to the administration period, the general toxicity of 1.2% DEHP was evident throughout the experimental period, and the genotoxicity of 10 p.p.m. DEN could be detected after 2 weeks of administration and further increased at 4 weeks. These results suggested that combined assays using gpt delta rats could detect both general toxicity and genotoxicity by the canonical 4-week administration protocol. Therefore, this assay using gpt delta rats would be applicable for risk assessment including early detection of genotoxic carcinogens and ultimately serve to reduce cancer risks in humans from environmental chemicals.
Keywords: Genotoxicity; gpt delta; mutation; reduction of animal use; repeated-dose toxicity.
© 2015 The Authors. Cancer Science published by Wiley Publishing Asia Pty Ltd on behalf of Japanese Cancer Association.
Figures




References
-
- OECD. OECD Guideline for Testing of Chemicals No. 488: Transgenic Rodent Somatic and Germ Cell Gene Mutation Assays. OECD: Paris; 2013.
-
- Hayashi H, Kondo H, Masumura K, Shindo Y, Nohmi T. Novel transgenic rat for in vivo genotoxicity assays using 6-thioguanine and Spi− selection. Environ Mol Mutagen. 2003;41:253–9. - PubMed
-
- Nohmi T, Katoh M, Suzuki H, et al. A new transgenic mouse mutagenesis test system using Spi− and 6-thioguanine selections. Environ Mol Mutagen. 1996;28:465–70. - PubMed
-
- Tatematsu M, Tsuda H, Shirai T, Masui T, Ito N. Placental glutathione S-transferase (GST-P) as a new marker for hepatocarcinogenesis: in vivo short-term screening for hepatocarcinogens. Toxicol Pathol. 1987;15:60–8. - PubMed
-
- IARC. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans: Some N-nitroso compounds. IARC: Lyon; 1977.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

