Neuroprotective role of Nrf2 for retinal ganglion cells in ischemia-reperfusion
- PMID: 25683606
- PMCID: PMC4413918
- DOI: 10.1111/jnc.13064
Neuroprotective role of Nrf2 for retinal ganglion cells in ischemia-reperfusion
Abstract
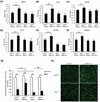
Retinal ischemia plays a critical role in multiple vision-threatening diseases and leads to death of retinal neurons, particularly ganglion cells. Oxidative stress plays an important role in this ganglion cell loss. Nrf2 (NF-E2-related factor 2) is a major regulator of the antioxidant response, and its role in the retina is increasingly appreciated. We investigated the potential retinal neuroprotective function of Nrf2 after ischemia-reperfusion (I/R) injury. In an experimental model of retinal I/R, Nrf2 knockout mice exhibited much greater loss of neuronal cells in the ganglion cell layer than wild-type mice. Primary retinal ganglion cells isolated from Nrf2 knockout mice exhibited decreased cell viability compared to wild-type retinal ganglion cells, demonstrating the cell-intrinsic protective role of Nrf2. The retinal neuronal cell line 661W exhibited reduced cell viability following siRNA-mediated knockdown of Nrf2 under conditions of oxidative stress, and this was associated with exacerbation of increase in reactive oxygen species. The synthetic triterpenoid CDDO-Im (2-Cyano-3,12-dioxooleana-1,9-dien-28-imidazolide), a potent Nrf2 activator, inhibited reactive oxygen species increase in cultured 661W under oxidative stress conditions and increased neuronal cell survival after I/R injury in wild-type, but not Nrf2 knockout mice. Our findings indicate that Nrf2 exhibits a retinal neuroprotective function in I/R and suggest that pharmacologic activation of Nrf2 could be a therapeutic strategy. Oxidative stress is thought to be an important mediator of retinal ganglion cell death in ischemia-reperfusion injury. We found that the transcription factor NF-E2-related factor 2 (Nrf2), a major regulator of oxidative stress, is an important endogenous neuroprotective molecule in retinal ganglion cells in ischemia-reperfusion, exerting a cell-autonomous protective effect. The triterpenoid 2-Cyano-3,12-dioxooleana-1,9-dien-28-imidazolide (CDDO-Im) reduces neurodegeneration following ischemia-reperfusion in an Nrf2-dependent fashion. This suggests that Nrf2-activating drugs including triterpenoids could be a therapeutic strategy for retinal neuroprotection.
Keywords: Nrf2; ganglion cell; ischemia; oxidative stress; retina.
© 2015 International Society for Neurochemistry.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures

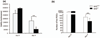


Similar articles
-
The Nrf2 Signaling in Retinal Ganglion Cells under Oxidative Stress in Ocular Neurodegenerative Diseases.Int J Biol Sci. 2018 Jun 8;14(9):1090-1098. doi: 10.7150/ijbs.25996. eCollection 2018. Int J Biol Sci. 2018. PMID: 29989056 Free PMC article. Review.
-
Critical role of Nrf2 in oxidative stress-induced retinal ganglion cell death.J Neurochem. 2013 Dec;127(5):669-80. doi: 10.1111/jnc.12325. Epub 2013 Jun 17. J Neurochem. 2013. PMID: 23721546
-
Monomethyl fumarate promotes Nrf2-dependent neuroprotection in retinal ischemia-reperfusion.J Neuroinflammation. 2015 Dec 21;12:239. doi: 10.1186/s12974-015-0452-z. J Neuroinflammation. 2015. PMID: 26689280 Free PMC article.
-
The triterpenoid CDDO-imidazolide ameliorates mouse liver ischemia-reperfusion injury through activating the Nrf2/HO-1 pathway enhanced autophagy.Cell Death Dis. 2017 Aug 10;8(8):e2983. doi: 10.1038/cddis.2017.386. Cell Death Dis. 2017. PMID: 28796242 Free PMC article.
-
[Retinal neuronal cell death: molecular mechanism and neuroprotection].Nippon Ganka Gakkai Zasshi. 2001 Dec;105(12):884-902. Nippon Ganka Gakkai Zasshi. 2001. PMID: 11802459 Review. Japanese.
Cited by
-
Nutrigenetic reprogramming of oxidative stress.Taiwan J Ophthalmol. 2021 Apr 30;11(3):207-215. doi: 10.4103/tjo.tjo_4_21. eCollection 2021 Jul-Sep. Taiwan J Ophthalmol. 2021. PMID: 34703735 Free PMC article. Review.
-
Age related retinal Ganglion cell susceptibility in context of autophagy deficiency.Cell Death Discov. 2020 Apr 17;6:21. doi: 10.1038/s41420-020-0257-4. eCollection 2020. Cell Death Discov. 2020. PMID: 32337073 Free PMC article.
-
The Nrf2 Signaling in Retinal Ganglion Cells under Oxidative Stress in Ocular Neurodegenerative Diseases.Int J Biol Sci. 2018 Jun 8;14(9):1090-1098. doi: 10.7150/ijbs.25996. eCollection 2018. Int J Biol Sci. 2018. PMID: 29989056 Free PMC article. Review.
-
Retinal oxidative stress activates the NRF2/ARE pathway: An early endogenous protective response to ocular hypertension.Redox Biol. 2021 Jun;42:101883. doi: 10.1016/j.redox.2021.101883. Epub 2021 Jan 29. Redox Biol. 2021. PMID: 33579667 Free PMC article.
-
A Mouse Model of Retinal Ischemia-Reperfusion Injury Through Elevation of Intraocular Pressure.J Vis Exp. 2016 Jul 14;(113):54065. doi: 10.3791/54065. J Vis Exp. 2016. PMID: 27501124 Free PMC article.
References
-
- Bastianetto S, Quirion R. Natural antioxidants and neurodegenerative diseases. Frontiers in bioscience : a journal and virtual library. 2004;9:3447–3452. - PubMed
-
- Gan L, Johnson JA. Oxidative damage and the Nrf2-ARE pathway in neurodegenerative diseases. Biochimica et biophysica acta. 2014;1842:1208–1218. - PubMed
-
- Himori N, Yamamoto K, Maruyama K, Ryu M, Taguchi K, Yamamoto M, Nakazawa T. Critical role of Nrf2 in oxidative stress-induced retinal ganglion cell death. Journal of neurochemistry. 2013;127:669–680. - PubMed
-
- Inoue Y, Shimazawa M, Nakamura S, Imamura T, Sugitani S, Tsuruma K, Hara H. Protective effects of placental growth factor on retinal neuronal cell damage. Journal of neuroscience research. 2014;92:329–337. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

