Interferon alpha (IFNα)-induced TRIM22 interrupts HCV replication by ubiquitinating NS5A
- PMID: 25683609
- PMCID: PMC4711679
- DOI: 10.1038/cmi.2014.131
Interferon alpha (IFNα)-induced TRIM22 interrupts HCV replication by ubiquitinating NS5A
Abstract
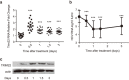
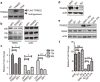
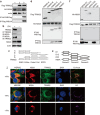
TRIM22, a tripartite-motif (TRIM) protein, is upregulated upon interferon alpha (IFNα) administration to hepatitis C virus (HCV)-infected patients. However, the physiological role of TRIM22 upregulation remains unclear. Here, we describe a potential antiviral function of TRIM22's targeting of the HCV NS5A protein. NS5A is important for HCV replication and for resistance to IFNα therapy. During the first 24 h following the initiation of IFNα treatment, upregulation of TRIM22 in the peripheral blood mononuclear cells (PBMCs) of HCV patients correlated with a decrease in viral titer. This phenomenon was confirmed in the hepatocyte-derived cell line Huh-7, which is highly permissive for HCV infection. TRIM22 over-expression inhibited HCV replication, and Small interfering RNA (siRNA)-mediated knockdown of TRIM22 diminished IFNα-induced anti-HCV function. Furthermore, we determined that TRIM22 ubiquitinates NS5A in a concentration-dependent manner. In summary, our results suggest that TRIM22 upregulation is associated with HCV decline during IFNα treatment and plays an important role in controlling HCV replication in vitro.
Figures


Similar articles
-
Associations between human TRIM22 gene expression and the response to combination therapy with Peg-IFNα-2a and ribavirin in Iranian patients with chronic hepatitis C.J Med Virol. 2014 Sep;86(9):1499-506. doi: 10.1002/jmv.23985. Epub 2014 May 29. J Med Virol. 2014. PMID: 24889558
-
Expression of TRIM22 mRNA in chronic hepatitis C patients treated with direct-acting antiviral drugs.APMIS. 2020 Apr;128(4):326-334. doi: 10.1111/apm.13024. Epub 2020 Jan 28. APMIS. 2020. PMID: 31863490
-
Increased expression and dysregulated association of restriction factors and type I interferon in HIV, HCV mono- and co-infected patients.J Med Virol. 2016 Jun;88(6):987-95. doi: 10.1002/jmv.24419. Epub 2015 Nov 9. J Med Virol. 2016. PMID: 26519943
-
New targets for antiviral therapy of chronic hepatitis C.Liver Int. 2012 Feb;32 Suppl 1:9-16. doi: 10.1111/j.1478-3231.2011.02701.x. Liver Int. 2012. PMID: 22212566 Review.
-
The interferon-stimulated gene TRIM22: A double-edged sword in HIV-1 infection.Cytokine Growth Factor Rev. 2018 Apr;40:40-47. doi: 10.1016/j.cytogfr.2018.02.001. Epub 2018 Feb 10. Cytokine Growth Factor Rev. 2018. PMID: 29650252 Review.
Cited by
-
TRIMming Type I Interferon-Mediated Innate Immune Response in Antiviral and Antitumor Defense.Viruses. 2021 Feb 11;13(2):279. doi: 10.3390/v13020279. Viruses. 2021. PMID: 33670221 Free PMC article. Review.
-
Neuralized E3 Ubiquitin Protein Ligase 3 Is an Inducible Antiviral Effector That Inhibits Hepatitis C Virus Assembly by Targeting Viral E1 Glycoprotein.J Virol. 2018 Oct 12;92(21):e01123-18. doi: 10.1128/JVI.01123-18. Print 2018 Nov 1. J Virol. 2018. PMID: 30111563 Free PMC article.
-
Junctional and somatic hypermutation-induced CX4C motif is critical for the recognition of a highly conserved epitope on HCV E2 by a human broadly neutralizing antibody.Cell Mol Immunol. 2021 Mar;18(3):675-685. doi: 10.1038/s41423-020-0403-1. Epub 2020 Mar 31. Cell Mol Immunol. 2021. PMID: 32235917 Free PMC article.
-
Interferons command Trim22 to fight against viruses.Cell Mol Immunol. 2017 Sep;14(9):794-796. doi: 10.1038/cmi.2017.76. Epub 2017 Aug 7. Cell Mol Immunol. 2017. PMID: 28782753 Free PMC article. No abstract available.
-
TRIM22 governs tumorigenesis and protects against endometrial cancer-associated cachexia by inhibiting inflammatory response and adipose thermogenic activity.Cancer Metab. 2025 Apr 8;13(1):17. doi: 10.1186/s40170-025-00386-2. Cancer Metab. 2025. PMID: 40200303 Free PMC article.
References
-
- 1Slomski A. WHO issues guidelines on HCV amid drug cost controversy. JAMA 2014; 311: 2262–2263. - PubMed
-
- 2Chevaliez S, Pawlotsky JM. HCV genome and life cycle. In: Tan SL, editor. Hepatitis C Viruses: Genomes and Molecular Biology. Norfolk: Horizon Bioscience, 2006. - PubMed
-
- 3Suzuki T, Aizaki H, Murakami K, Shoji I, Wakita T. Molecular biology of hepatitis C virus. J Gastroenterol 2007; 42: 411–423. - PubMed
-
- 4Levrero M. Viral hepatitis and liver cancer: the case of hepatitis C. Oncogene 2006; 25: 3834–3847. - PubMed
-
- 5Lai CL. Antiviral therapy for hepatitis B and C in Asians. J Gastroenterol Hepatol 1999; 14 Suppl: S19–S21. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

