Discovery and SAR of novel series of imidazopyrimidinones and dihydroimidazopyrimidinones as positive allosteric modulators of the metabotropic glutamate receptor 5 (mGlu5)
- PMID: 25683622
- PMCID: PMC4399811
- DOI: 10.1016/j.bmcl.2015.01.038
Discovery and SAR of novel series of imidazopyrimidinones and dihydroimidazopyrimidinones as positive allosteric modulators of the metabotropic glutamate receptor 5 (mGlu5)
Abstract
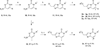
We report the discovery and SAR of two novel series of imidazopyrimidinones and dihydroimidazopyrimidinones as metabotropic glutamate receptor 5 (mGlu5) positive allosteric modulators (PAMs). Exploration of several structural features in the western and eastern part of the imidazopyrimidinone core and combinations thereof, revealed compound 4a as a mGlu5 PAM with good in vitro potency and efficacy, acceptable drug metabolism and pharmacokinetic (DMPK) properties and in vivo efficacy in an amphetamine-based model of psychosis. However, the presence of CNS-mediated adverse effects in preclinical species precluded any further in vivo evaluation.
Keywords: Dihydroimidazopyrimidinone; Imidazopyrimidinone; Metabotropic glutamate receptor 5 (mGlu(5)); Positive allosteric modulator (PAM); Schizophrenia.
Copyright © 2015 Elsevier Ltd. All rights reserved.
Figures




Similar articles
-
Identification and optimisation of a series of tetrahydrobenzotriazoles as metabotropic glutamate receptor 5-selective positive allosteric modulators that improve performance in a preclinical model of cognition.Bioorg Med Chem Lett. 2015 Dec 15;25(24):5792-6. doi: 10.1016/j.bmcl.2015.10.050. Epub 2015 Oct 17. Bioorg Med Chem Lett. 2015. PMID: 26531152
-
Tetrahydronaphthyridine and dihydronaphthyridinone ethers as positive allosteric modulators of the metabotropic glutamate receptor 5 (mGlu₅).J Med Chem. 2014 Jul 10;57(13):5620-37. doi: 10.1021/jm500259z. Epub 2014 Jun 25. J Med Chem. 2014. PMID: 24914612 Free PMC article.
-
Dihydrothiazolopyridone derivatives as a novel family of positive allosteric modulators of the metabotropic glutamate 5 (mGlu5) receptor.J Med Chem. 2013 Sep 26;56(18):7243-59. doi: 10.1021/jm400650w. Epub 2013 Sep 4. J Med Chem. 2013. PMID: 23947773 Free PMC article.
-
SAR studies on mGlu5 receptor positive allosteric modulators (2003- 2013).Curr Top Med Chem. 2014;14(15):1789-841. doi: 10.2174/1568026614666140826120419. Curr Top Med Chem. 2014. PMID: 25176124 Review.
-
Progress toward positive allosteric modulators of the metabotropic glutamate receptor subtype 5 (mGluR5).ACS Chem Neurosci. 2011 Aug 17;2(8):450-70. doi: 10.1021/cn2000519. Epub 2011 Jun 27. ACS Chem Neurosci. 2011. PMID: 22860171 Free PMC article. Review.
Cited by
-
Further optimization of the M1 PAM VU0453595: Discovery of novel heterobicyclic core motifs with improved CNS penetration.Bioorg Med Chem Lett. 2016 Aug 1;26(15):3822-5. doi: 10.1016/j.bmcl.2016.04.083. Epub 2016 Apr 29. Bioorg Med Chem Lett. 2016. PMID: 27173801 Free PMC article.
-
Acyl dihydropyrazolo[1,5-a]pyrimidinones as metabotropic glutamate receptor 5 positive allosteric modulators.Bioorg Med Chem Lett. 2015 Nov 15;25(22):5115-20. doi: 10.1016/j.bmcl.2015.10.009. Epub 2015 Oct 9. Bioorg Med Chem Lett. 2015. PMID: 26475522 Free PMC article.
-
A Novel Phenylpyrrolidine Derivative: Synthesis and Effect on Cognitive Functions in Rats with Experimental Ishemic Stroke.Molecules. 2021 Oct 11;26(20):6124. doi: 10.3390/molecules26206124. Molecules. 2021. PMID: 34684709 Free PMC article.
-
Preliminary investigation of 6,7-dihydropyrazolo[1,5-a]pyrazin-4-one derivatives as a novel series of mGlu5 receptor positive allosteric modulators with efficacy in preclinical models of schizophrenia.Bioorg Med Chem Lett. 2016 Jan 15;26(2):429-434. doi: 10.1016/j.bmcl.2015.11.098. Epub 2015 Nov 28. Bioorg Med Chem Lett. 2016. PMID: 26684851 Free PMC article.
-
Discovery of VU0409551/JNJ-46778212: An mGlu5 Positive Allosteric Modulator Clinical Candidate Targeting Schizophrenia.ACS Med Chem Lett. 2015 May 20;6(6):716-20. doi: 10.1021/acsmedchemlett.5b00181. eCollection 2015 Jun 11. ACS Med Chem Lett. 2015. PMID: 26157544 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information
Miscellaneous

