The interaction between maternal immune activation and alpha 7 nicotinic acetylcholine receptor in regulating behaviors in the offspring
- PMID: 25683697
- PMCID: PMC4414803
- DOI: 10.1016/j.bbi.2015.02.005
The interaction between maternal immune activation and alpha 7 nicotinic acetylcholine receptor in regulating behaviors in the offspring
Abstract
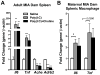
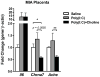
Mutation of human chromosome 15q13.3 increases the risk for autism and schizophrenia. One of the noteworthy genes in 15q13.3 is CHRNA7, which encodes the nicotinic acetylcholine receptor alpha 7 subunit (α7nAChR) associated with schizophrenia in clinical studies and rodent models. This study investigates the role of α7nAChR in maternal immune activation (MIA) mice model, a murine model of environmental risk factor for autism and schizophrenia. We provided choline, a selective α7nAChR agonist among its several developmental roles, in the diet of C57BL/6N wild-type dams throughout the gestation and lactation period and induced MIA at mid-gestation. The adult offspring behavior and gene expression profile in the maternal-placental-fetal axis at mid-gestation were investigated. We found that choline supplementation prevented several MIA-induced behavioral abnormalities in the wild-type offspring. Pro-inflammatory cytokine interleukin-6 (Il6) and Chrna7 gene expression in the wild-type fetal brain were elevated by poly(I:C) injection and were suppressed by gestational choline supplementation. We further investigated the gene expression level of Il6 in Chrna7 mutant mice. We found that the basal level of Il6 was higher in Chrna7 mutant fetal brain, which suggests that α7nAChR may serve an anti-inflammatory role in the fetal brain during development. Lastly, we induced MIA in Chrna7(+/-) offspring. The Chrna7(+/-) offspring were more vulnerable to MIA, with increased behavioral abnormalities. Our study shows that α7nAChR modulates inflammatory response affecting the fetal brain and demonstrates its effects on offspring behavior development after MIA.
Keywords: Autism; Choline supplementation; Interleukin-6 (IL-6); Maternal immune activation (MIA); Maternal infection; Nicotinic acetylcholine receptor alpha 7 subunit (α7AChR, CHRNA7, Chrna7); Poly(I:C); Schizophrenia.
Copyright © 2015 Elsevier Inc. All rights reserved.
Conflict of interest statement
All authors declare that there are no conflicts of interest.
Figures






References
-
- Abazyan B, Nomura J, Kannan G, Ishizuka K, Tamashiro KL, Nucifora F, Pogorelov V, Ladenheim B, Yang C, Krasnova IN, Cadet JL, Pardo C, Mori S, Kamiya A, Vogel MW, Sawa A, Ross CA, Pletnikov MV. Prenatal interaction of mutant DISC1 and immune activation produces adult psychopathology. Biol Psychiatry. 2010;68:1172–1181. - PMC - PubMed
-
- Adams CE, Broide RS, Chen Y, Winzer-Serhan UH, Henderson TA, Leslie FM, Freedman R. Development of the alpha7 nicotinic cholinergic receptor in rat hippocampal formation. Brain Res Dev Brain Res. 2002;139:175–187. - PubMed
-
- Adams CE, Stevens KE. Evidence for a role of nicotinic acetylcholine receptors in schizophrenia. Front Biosci. 2007;12:4755–4772. - PubMed
-
- Alkondon M, Pereira EF, Cortes WS, Maelicke A, Albuquerque EX. Choline is a selective agonist of alpha7 nicotinic acetylcholine receptors in the rat brain neurons. Eur J Neurosci. 1997;9:2734–2742. - PubMed
-
- Azzopardi E, Typlt M, Jenkins B, Schmid S. Sensorimotor gating and spatial learning in alpha7-nicotinic receptor knockout mice. Genes Brain Behav. 2013;12:414–423. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

