Spontaneous Neurotransmitter Release Shapes Dendritic Arbors via Long-Range Activation of NMDA Receptors
- PMID: 25683710
- PMCID: PMC4542315
- DOI: 10.1016/j.celrep.2015.01.032
Spontaneous Neurotransmitter Release Shapes Dendritic Arbors via Long-Range Activation of NMDA Receptors
Abstract
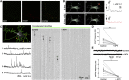
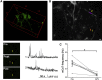
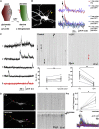
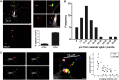
Spontaneous neurotransmitter release is a core element of synaptic communication in mature neurons, but despite exceptionally high levels of spontaneous vesicle cycling occurring in developing axons, little is known of its function during this period. We now show that high-level, spontaneous axonal release of the neurotransmitter glutamate can signal at long range to NMDA receptors on developing dendrites, prior to synapse formation and, indeed, axodendritic contact. Blockade of NMDA signaling during this early period of spontaneous vesicle cycling leads to a reduction in dendritic arbor complexity, indicating an important role for early spontaneous release in dendritic arbor growth.
Copyright © 2015 The Authors. Published by Elsevier Inc. All rights reserved.
Figures

References
-
- Akashi K., Kakizaki T., Kamiya H., Fukaya M., Yamasaki M., Abe M., Natsume R., Watanabe M., Sakimura K. NMDA receptor GluN2B (GluR epsilon 2/NR2B) subunit is crucial for channel function, postsynaptic macromolecular organization, and actin cytoskeleton at hippocampal CA3 synapses. J. Neurosci. 2009;29:10869–10882. - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

