Improved emergency myelopoiesis and survival in neonatal sepsis by caspase-1/11 ablation
- PMID: 25684123
- PMCID: PMC4427394
- DOI: 10.1111/imm.12450
Improved emergency myelopoiesis and survival in neonatal sepsis by caspase-1/11 ablation
Abstract
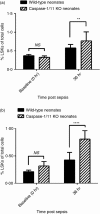
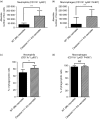
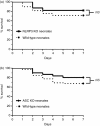
Over one million newborns die annually from sepsis with the highest mortality in premature and low-birthweight infants. The inflammasome plays a central role in the regulation of innate immunity and inflammation, and is presumed to be involved in protective immunity, in large part through the caspase-1-dependent activation of interleukin-1β (IL-1β) and IL-18. Studies in endotoxic shock, however, suggest that endogenous caspase-1 activity and the inflammasome contribute to mortality primarily by promoting excessive systemic inflammatory responses. We examined whether caspase-1 and the inflammasome also regulate neonatal inflammation, host protective immunity and myelopoiesis during polymicrobial sepsis. Neonatal (5-7 days) C57BL/6 and caspase-1/11(-/-) mice underwent a low-lethality caecal slurry model of intra-abdominal sepsis (LD25-45 ). Ablation of caspase-1/11, but not apoptosis-associated speck-like protein containing a CARD domain or nucleotide-binding oligomerization domain-like receptor protein 3 (NLRP3), improved neonatal survival following septic challenge compared with wild-type mice (P < 0·001), with decreased concentrations of inflammatory cytokines in the serum and peritoneum. Surprisingly, caspase-1/11(-/-) neonates also exhibited increased bone marrow and splenic haematopoietic stem cell expansion (P < 0·001), and increased concentrations of granulocyte and macrophage colony-stimulating factors in the peritoneum (P < 0·001) after sepsis. Ablation of caspase-1/11 signalling was also associated with increased recruitment of peritoneal macrophages and neutrophils (P < 0·001), increased phagocytosis by neutrophils (P = 0·003), and decreased bacterial colonization (P = 0·02) in the peritoneum. These findings suggest that endogenous caspase-1/11 activity, independent of the NLRP3 inflammasome, not only promotes the magnitude of the inflammatory response, but also suppresses protective immunity in the neonate, so contributing to innate immune dysfunction and poor survival in neonatal sepsis.
Keywords: caspase-1/11; emergency myelopoiesis; inflammation; mouse; neonates.
© 2015 John Wiley & Sons Ltd.
Figures



References
-
- Watson RS, Carcillo JA, Linde-Zwirble WT, Clermont G, Lidicker J, Angus DC. The epidemiology of severe sepsis in children in the United States. Am J Respir Crit Care Med. 2003;167:695–701. - PubMed
-
- Adkins B, Leclerc C, Marshall-Clarke S. Neonatal adaptive immunity comes of age. Nat Rev Immunol. 2004;4:553–64. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

