α-Lipoic acid protects against hypoxia/reoxygenation-induced injury in human umbilical vein endothelial cells through suppression of apoptosis and autophagy
- PMID: 25684163
- PMCID: PMC4438966
- DOI: 10.3892/mmr.2015.3351
α-Lipoic acid protects against hypoxia/reoxygenation-induced injury in human umbilical vein endothelial cells through suppression of apoptosis and autophagy
Abstract
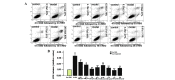
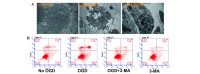
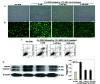
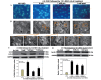
α-Lipoic acid (ALA) is known as a powerful antioxidant, which has been reported to have protective effects against various cardiovascular diseases. The present study aimed to determine whether ALA pre- or post-treatment induced protective effects against hypoxia/reoxygenation-induced injury via inhibition of apoptosis and autophagy in human umbilical vein endothelial cells (HUVECs). In order to simulate the conditions of hypoxia/reoxygenation, HUVECs were subjected to 4 h of oxygen-glucose deprivation (OGD) followed by 12 h of reoxygenation. For the pre-treatment, ALA was added to the buffer 12 h prior to OGD, whereas for the post-treatment, ALA was added at the initiation of reoxygenation. The results demonstrated that ALA pre- or post-treatment significantly reduced lactate dehydrogenase (LDH) release induced through hypoxia/reoxygenation in HUVECs in a dose-dependent manner; of note, 1 mM ALA pre- or post-treatment exhibited the most potent protective effects. In addition, ALA significantly reduced hypoxia/reoxygenation-induced loss of mitochondrial membrane potential, apoptosis and the expression of cleaved caspase-3 in HUVECs. In the presence of the specific autophagy inhibitor 3-methyladenine, hypoxia/reoxygenation-induced apoptosis was significantly reduced. Furthermore, the formation of autophagosomes, cytosolic microtubule-associated protein 1A/1B-light chain 3 ratio and beclin1 levels significantly increased following hypoxia/reoxygenation injury; however, all of these effects were ameliorated following pre- or post-treatment with ALA. The results of the present study suggested that ALA may provide beneficial protection against hypoxia/reoxygenation-induced injury via attenuation of apoptosis and autophagy in HUVECs.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

