Isoaspartate, carbamoyl phosphate synthase-1, and carbonic anhydrase-III as biomarkers of liver injury
- PMID: 25684186
- PMCID: PMC4355035
- DOI: 10.1016/j.bbrc.2015.01.158
Isoaspartate, carbamoyl phosphate synthase-1, and carbonic anhydrase-III as biomarkers of liver injury
Abstract
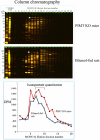
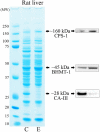
We had previously shown that alcohol consumption can induce cellular isoaspartate protein damage via an impairment of the activity of protein isoaspartyl methyltransferase (PIMT), an enzyme that triggers repair of isoaspartate protein damage. To further investigate the mechanism of isoaspartate accumulation, hepatocytes cultured from control or 4-week ethanol-fed rats were incubated in vitro with tubercidin or adenosine. Both these agents, known to elevate intracellular S-adenosylhomocysteine levels, increased cellular isoaspartate damage over that recorded following ethanol consumption in vivo. Increased isoaspartate damage was attenuated by treatment with betaine. To characterize isoaspartate-damaged proteins that accumulate after ethanol administration, rat liver cytosolic proteins were methylated using exogenous PIMT and (3)H-S-adenosylmethionine and proteins resolved by gel electrophoresis. Three major protein bands of ∼ 75-80 kDa, ∼ 95-100 kDa, and ∼ 155-160 kDa were identified by autoradiography. Column chromatography used to enrich isoaspartate-damaged proteins indicated that damaged proteins from ethanol-fed rats were similar to those that accrued in the livers of PIMT knockout (KO) mice. Carbamoyl phosphate synthase-1 (CPS-1) was partially purified and identified as the ∼ 160 kDa protein target of PIMT in ethanol-fed rats and in PIMT KO mice. Analysis of the liver proteome of 4-week ethanol-fed rats and PIMT KO mice demonstrated elevated cytosolic CPS-1 and betaine homocysteine S-methyltransferase-1 when compared to their respective controls, and a significant reduction of carbonic anhydrase-III (CA-III) evident only in ethanol-fed rats. Ethanol feeding of rats for 8 weeks resulted in a larger (∼ 2.3-fold) increase in CPS-1 levels compared to 4-week ethanol feeding indicating that CPS-1 accumulation correlated with the duration of ethanol consumption. Collectively, our results suggest that elevated isoaspartate and CPS-1, and reduced CA-III levels could serve as biomarkers of hepatocellular injury.
Keywords: Alcohol-induced liver injury; Carbamoyl phosphate synthase-1; Carbonic anhydrase-III; Isoaspartate; Liver proteome; Protein isoaspartyl methyltransferase.
Copyright © 2015 The Authors. Published by Elsevier Inc. All rights reserved.
Figures





References
-
- Global status report on alcohol and health 2014. World Health Organization; Geneva: 2014. http://www.who.int/substance_abuse/publications/global_alcohol_report/en/
-
- Cederbaum AI, Lu Y, Wu D. Role of oxidative stress in alcohol-induced liver injury. Arch Toxicol. 2009;83:519–548. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

