Inhibition of Pro-apoptotic BAX by a noncanonical interaction mechanism
- PMID: 25684204
- PMCID: PMC4384643
- DOI: 10.1016/j.molcel.2015.01.014
Inhibition of Pro-apoptotic BAX by a noncanonical interaction mechanism
Abstract
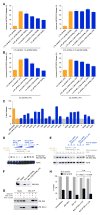
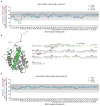
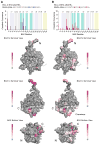
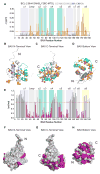
BCL-2 is a negative regulator of apoptosis implicated in homeostatic and pathologic cell survival. The canonical anti-apoptotic mechanism involves entrapment of activated BAX by a groove on BCL-2, preventing BAX homo-oligomerization and mitochondrial membrane poration. The BCL-2 BH4 domain also confers anti-apoptotic functionality, but the mechanism is unknown. We find that a synthetic α-helical BH4 domain binds to BAX with nanomolar affinity and independently inhibits the conformational activation of BAX. Hydrogen-deuterium exchange mass spectrometry demonstrated that the N-terminal conformational changes in BAX induced by a triggering BIM BH3 helix were suppressed by the BCL-2 BH4 helix. Structural analyses localized the BH4 interaction site to a groove formed by residues of α1, α1-α2 loop, and α2-α3 and α5-α6 hairpins on the BAX surface. These data reveal a previously unappreciated binding site for targeted inhibition of BAX and suggest that the BCL-2 BH4 domain may participate in apoptosis blockade by a noncanonical interaction mechanism.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures



References
-
- Czabotar PE, Westphal D, Dewson G, Ma S, Hockings C, Fairlie WD, Lee EF, Yao S, Robin AY, Smith BJ, et al. Bax crystal structures reveal how BH3 domains activate Bax and nucleate its oligomerization to induce apoptosis. Cell. 2013;152:519–531. - PubMed
-
- Ding J, Mooers BH, Zhang Z, Kale J, Falcone D, McNichol J, Huang B, Zhang XC, Xing C, Andrews DW, Lin J. After embedding in membranes antiapoptotic Bcl-XL protein binds both Bcl-2 homology region 3 and helix 1 of proapoptotic Bax protein to inhibit apoptotic mitochondrial permeabilization. J Biol Chem. 2014;289:11873–11896. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R00HL095929/HL/NHLBI NIH HHS/United States
- R00 HL095929/HL/NHLBI NIH HHS/United States
- T32GM007306/GM/NIGMS NIH HHS/United States
- R01 GM086507/GM/NIGMS NIH HHS/United States
- 5R01GM090299/GM/NIGMS NIH HHS/United States
- R01 CA178394/CA/NCI NIH HHS/United States
- R01 GM101135/GM/NIGMS NIH HHS/United States
- T32 GM007306/GM/NIGMS NIH HHS/United States
- R01GM101135/GM/NIGMS NIH HHS/United States
- R01GM086507/GM/NIGMS NIH HHS/United States
- R01 GM090299/GM/NIGMS NIH HHS/United States
- R01 CA050239/CA/NCI NIH HHS/United States
- P30 CA013330/CA/NCI NIH HHS/United States
- 5R01CA050239/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

