Thalamic Kv 7 channels: pharmacological properties and activity control during noxious signal processing
- PMID: 25684311
- PMCID: PMC4459028
- DOI: 10.1111/bph.13113
Thalamic Kv 7 channels: pharmacological properties and activity control during noxious signal processing
Abstract
Background and purpose: The existence of functional K(v)7 channels in thalamocortical (TC) relay neurons and the effects of the K(+)-current termed M-current (I(M)) on thalamic signal processing have long been debated. Immunocytochemical evidence suggests their presence in this brain region. Therefore, we aimed to verify their existence, pharmacological properties and function in regulating activity in neurons of the ventrobasal thalamus (VB).
Experimental approach: Characterization of K(v)7 channels was performed by combining in vitro, in vivo and in silico techniques with a pharmacological approach. Retigabine (30 μM) and XE991 (20 μM), a specific K(v)7 channel enhancer and blocker, respectively, were applied in acute brain slices during electrophysiological recordings. The effects of intrathalamic injection of retigabine (3 mM, 300 nL) and/or XE991 (2 mM, 300 nL) were investigated in freely moving animals during hot-plate tests by recording behaviour and neuronal activity.
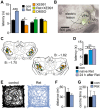
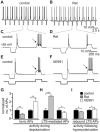
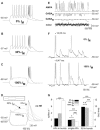
Key results: K(v)7.2 and K(v)7.3 subunits were found to be abundantly expressed in TC neurons of mouse VB. A slow K(+)-current with properties of IM was activated by retigabine and inhibited by XE991. K(v)7 channel activation evoked membrane hyperpolarization, a reduction in tonic action potential firing, and increased burst firing in vitro and in computational models. Single-unit recordings and pharmacological intervention demonstrated a specific burst-firing increase upon I(M) activation in vivo. A K(v)7 channel-mediated increase in pain threshold was associated with fewer VB units responding to noxious stimuli, and increased burst firing in responsive neurons.
Conclusions and implications: K(v)7 channel enhancement alters somatosensory activity and may reflect an anti-nociceptive mechanism during acute pain processing.
© 2015 The British Pharmacological Society.
Figures



Similar articles
-
Kv7/KCNQ/M-channels in rat glutamatergic hippocampal axons and their role in regulation of excitability and transmitter release.J Physiol. 2006 Oct 1;576(Pt 1):235-56. doi: 10.1113/jphysiol.2006.111336. Epub 2006 Jul 13. J Physiol. 2006. PMID: 16840518 Free PMC article.
-
KCNQ/M-channels regulate mouse vagal bronchopulmonary C-fiber excitability and cough sensitivity.JCI Insight. 2019 Mar 7;4(5):e124467. doi: 10.1172/jci.insight.124467. eCollection 2019 Mar 7. JCI Insight. 2019. PMID: 30721152 Free PMC article.
-
Kv7/M channel dysfunction produces hyperexcitability in hippocampal CA1 pyramidal cells of Fmr1 knockout mice.J Physiol. 2024 Aug;602(15):3769-3791. doi: 10.1113/JP285244. Epub 2024 Jul 8. J Physiol. 2024. PMID: 38976504
-
The mechanism of action of retigabine (ezogabine), a first-in-class K+ channel opener for the treatment of epilepsy.Epilepsia. 2012 Mar;53(3):412-24. doi: 10.1111/j.1528-1167.2011.03365.x. Epub 2012 Jan 5. Epilepsia. 2012. PMID: 22220513 Review.
-
Pharmacological Targeting of Neuronal Kv7.2/3 Channels: A Focus on Chemotypes and Receptor Sites.Curr Med Chem. 2018;25(23):2637-2660. doi: 10.2174/0929867324666171012122852. Curr Med Chem. 2018. PMID: 29022505 Review.
Cited by
-
Flexible Stoichiometry: Implications for KCNQ2- and KCNQ3-Associated Neurodevelopmental Disorders.Dev Neurosci. 2021;43(3-4):191-200. doi: 10.1159/000515495. Epub 2021 Apr 1. Dev Neurosci. 2021. PMID: 33794528 Free PMC article. Review.
-
Orexin A as a modulator of dorsal lateral geniculate neuronal activity: a comprehensive electrophysiological study on adult rats.Sci Rep. 2019 Nov 13;9(1):16729. doi: 10.1038/s41598-019-53012-9. Sci Rep. 2019. PMID: 31723155 Free PMC article.
-
Voltage-Gated Ion Channels in Neuropathic Pain Signaling.Life (Basel). 2025 May 30;15(6):888. doi: 10.3390/life15060888. Life (Basel). 2025. PMID: 40566541 Free PMC article. Review.
-
Modulation of thalamocortical oscillations by TRIP8b, an auxiliary subunit for HCN channels.Brain Struct Funct. 2018 Apr;223(3):1537-1564. doi: 10.1007/s00429-017-1559-z. Epub 2017 Nov 22. Brain Struct Funct. 2018. PMID: 29168010 Free PMC article.
-
Local activation of CB1 receptors by synthetic and endogenous cannabinoids dampens burst firing mode of reticular thalamic nucleus neurons in rats under ketamine anesthesia.Exp Brain Res. 2024 Sep;242(9):2137-2157. doi: 10.1007/s00221-024-06889-6. Epub 2024 Jul 9. Exp Brain Res. 2024. PMID: 38980339
References
-
- Bezdudnaya T, Cano M, Bereshpolova Y, Stoelzel CR, Alonso J-M, Swadlow HA. Thalamic burst mode and inattention in the awake LGNd. Neuron. 2006;49:421–432. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

