Inhibiting endocannabinoid biosynthesis: a novel approach to the treatment of constipation
- PMID: 25684407
- PMCID: PMC4459026
- DOI: 10.1111/bph.13114
Inhibiting endocannabinoid biosynthesis: a novel approach to the treatment of constipation
Abstract
Background and purpose: Endocannabinoids are a family of lipid mediators involved in the regulation of gastrointestinal (GI) motility. The expression, localization and function of their biosynthetic enzymes in the GI tract are not well understood. Here, we examined the expression, localization and function of the enzyme diacylglycerol lipase-α (DAGLα), which is involved in biosynthesis of the endocannabinoid 2-arachidonoylglycerol (2-AG).
Experimental approach: Cannabinoid CB1 receptor-deficient, wild-type control and C3H/HeJ mice, a genetically constipated strain, were used. The distribution of DAGLα in the enteric nervous system was examined by immunohistochemistry. Effects of the DAGL inhibitors, orlistat and OMDM-188 on pharmacologically induced GI hypomotility were assessed by measuring intestinal contractility in vitro and whole gut transit or faecal output in vivo. Endocannabinoid levels were measured by mass spectrometry.
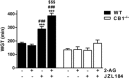
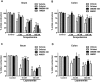
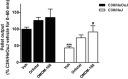
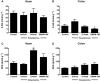
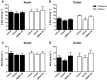
Key results: DAGLα was expressed throughout the GI tract. In the intestine, unlike DAGLβ, DAGLα immunoreactivity was prominently expressed in the enteric nervous system. In the myenteric plexus, it was colocalized with the vesicular acetylcholine transporter in cholinergic nerves. In normal mice, inhibiting DAGL reversed both pharmacologically reduced intestinal contractility and pharmacologically prolonged whole gut transit. Moreover, inhibiting DAGL normalized faecal output in constipated C3H/HeJ mice. In colons incubated with scopolamine, 2-AG was elevated while inhibiting DAGL normalized 2-AG levels.
Conclusions and implications: DAGLα was expressed in the enteric nervous system of mice and its inhibition reversed slowed GI motility, intestinal contractility and constipation through 2-AG and CB1 receptor-mediated mechanisms. Our data suggest that DAGLα inhibitors may be promising candidates for the treatment of constipation.
© 2015 The British Pharmacological Society.
Figures


References
-
- Abalo R, Vera G, López-Pérez AE, Martinez-Villaluenga M, Martín-Fontelles MI. The gastrointestinal pharmacology of cannabinoids: focus on motility. Pharmacology. 2012;90:1–10. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

