Menopausal hormone use and ovarian cancer risk: individual participant meta-analysis of 52 epidemiological studies
- PMID: 25684585
- PMCID: PMC4427760
- DOI: 10.1016/S0140-6736(14)61687-1
Menopausal hormone use and ovarian cancer risk: individual participant meta-analysis of 52 epidemiological studies
Abstract
Background: Half the epidemiological studies with information about menopausal hormone therapy and ovarian cancer risk remain unpublished, and some retrospective studies could have been biased by selective participation or recall. We aimed to assess with minimal bias the effects of hormone therapy on ovarian cancer risk.
Methods: Individual participant datasets from 52 epidemiological studies were analysed centrally. The principal analyses involved the prospective studies (with last hormone therapy use extrapolated forwards for up to 4 years). Sensitivity analyses included the retrospective studies. Adjusted Poisson regressions yielded relative risks (RRs) versus never-use.
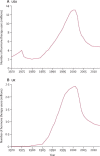
Findings: During prospective follow-up, 12 110 postmenopausal women, 55% (6601) of whom had used hormone therapy, developed ovarian cancer. Among women last recorded as current users, risk was increased even with <5 years of use (RR 1·43, 95% CI 1·31-1·56; p<0·0001). Combining current-or-recent use (any duration, but stopped <5 years before diagnosis) resulted in an RR of 1·37 (95% CI 1·29-1·46; p<0·0001); this risk was similar in European and American prospective studies and for oestrogen-only and oestrogen-progestagen preparations, but differed across the four main tumour types (heterogeneity p<0·0001), being definitely increased only for the two most common types, serous (RR 1·53, 95% CI 1·40-1·66; p<0·0001) and endometrioid (1·42, 1·20-1·67; p<0·0001). Risk declined the longer ago use had ceased, although about 10 years after stopping long-duration hormone therapy use there was still an excess of serous or endometrioid tumours (RR 1·25, 95% CI 1·07-1·46, p=0·005).
Interpretation: The increased risk may well be largely or wholly causal; if it is, women who use hormone therapy for 5 years from around age 50 years have about one extra ovarian cancer per 1000 users and, if its prognosis is typical, about one extra ovarian cancer death per 1700 users.
Funding: Medical Research Council, Cancer Research UK.
Copyright © 2015 Collaborative Group on Epidemiological Studies of Ovarian Cancer. Open Access article distributed under the terms of CC BY. Published by Elsevier Ltd. All rights reserved.
Figures



Comment in
-
Short term use of HRT increases risk of ovarian cancer, analysis finds.BMJ. 2015 Feb 13;350:h840. doi: 10.1136/bmj.h840. BMJ. 2015. PMID: 25681103 No abstract available.
-
Hormone therapy: short-term relief, long-term consequences.Lancet. 2015 May 9;385(9980):1806-8. doi: 10.1016/S0140-6736(14)62458-2. Epub 2015 Feb 13. Lancet. 2015. PMID: 25684588 No abstract available.
-
Risk factors: HRT increases risk of ovarian cancer.Nat Rev Clin Oncol. 2015 May;12(5):251. doi: 10.1038/nrclinonc.2015.44. Epub 2015 Mar 3. Nat Rev Clin Oncol. 2015. PMID: 25734633 No abstract available.
-
Reproductive endocrinology: Menopausal hormone therapy-ovarian cancer risk revisited.Nat Rev Endocrinol. 2015 Jun;11(6):322-3. doi: 10.1038/nrendo.2015.33. Epub 2015 Mar 10. Nat Rev Endocrinol. 2015. PMID: 25752281 No abstract available.
-
[HRT and ovarian cancer risk].Gynecol Obstet Fertil. 2015 Apr;43(4):324-5. doi: 10.1016/j.gyobfe.2015.02.015. Epub 2015 Mar 26. Gynecol Obstet Fertil. 2015. PMID: 25819391 French. No abstract available.
-
Hormone therapy and ovarian cancer.Lancet. 2015 Sep 12;386(9998):1037. doi: 10.1016/S0140-6736(15)00137-3. Lancet. 2015. PMID: 26382990 No abstract available.
-
Hormone therapy and ovarian cancer.Lancet. 2015 Sep 12;386(9998):1037-8. doi: 10.1016/S0140-6736(15)00138-5. Lancet. 2015. PMID: 26382991 No abstract available.
-
Hormone therapy and ovarian cancer.Lancet. 2015 Sep 12;386(9998):1038. doi: 10.1016/S0140-6736(15)00139-7. Lancet. 2015. PMID: 26382992 No abstract available.
-
Hormone therapy and ovarian cancer - Authors' reply.Lancet. 2015 Sep 12;386(9998):1038-9. doi: 10.1016/S0140-6736(15)00140-3. Lancet. 2015. PMID: 26382993 No abstract available.
References
-
- Writing Group for the Women's Health Initiative Investigators Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women's Health Initiative randomized controlled trial. JAMA. 2002;288:321–333. - PubMed
-
- European Medicines Agency Guidelines on clinical investigation of medicinal products for hormone replacement therapy of oestrogen deficiency symptoms in postmenopausal women. www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/... (accessed Jan 1, 2014).
-
- US Food and Drug Administration Estrogen and estrogen with progestin therapies for postmenopausal women. www.fda.gov/Drugs/DrugSafety/InformationbyDrugClass/ucm135318.htm (accessed Jan 1, 2014).
-
- Medicines and Healthcare products Regulatory Agency (MHRA) Hormone-replacement therapy: safety update. UK Public Assessment Report. www.mhra.gov.uk/home/groups/pl-p/documents/websiteresources/con2032228.pdf (accessed Jan 1, 2014).
-
- Beral V, Reeves G, Green J, Bull D. Ovarian cancer and hormone replacement therapy in the Million Women Study. Lancet. 2007;369:1703–1710. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

