Improvement of predictive models of risk of disease progression in chronic hepatitis C by incorporating longitudinal data
- PMID: 25684666
- PMCID: PMC4480773
- DOI: 10.1002/hep.27750
Improvement of predictive models of risk of disease progression in chronic hepatitis C by incorporating longitudinal data
Abstract
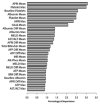
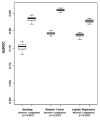
Existing predictive models of risk of disease progression in chronic hepatitis C have limited accuracy. The aim of this study was to improve upon existing models by applying novel statistical methods that incorporate longitudinal data. Patients in the Hepatitis C Antiviral Long-term Treatment Against Cirrhosis trial were analyzed. Outcomes of interest were (1) fibrosis progression (increase of two or more Ishak stages) and (2) liver-related clinical outcomes (liver-related death, hepatic decompensation, hepatocellular carcinoma, liver transplant, or increase in Child-Turcotte-Pugh score to ≥7). Predictors included longitudinal clinical, laboratory, and histologic data. Models were constructed using logistic regression and two machine learning methods (random forest and boosting) to predict an outcome in the next 12 months. The control arm was used as the training data set (n = 349 clinical, n = 184 fibrosis) and the interferon arm, for internal validation. The area under the receiver operating characteristic curve for longitudinal models of fibrosis progression was 0.78 (95% confidence interval [CI] 0.74-0.83) using logistic regression, 0.79 (95% CI 0.77-0.81) using random forest, and 0.79 (95% CI 0.77-0.82) using boosting. The area under the receiver operating characteristic curve for longitudinal models of clinical progression was 0.79 (95% CI 0.77-0.82) using logistic regression, 0.86 (95% CI 0.85-0.87) using random forest, and 0.84 (95% CI 0.82-0.86) using boosting. Longitudinal models outperformed baseline models for both outcomes (P < 0.0001). Longitudinal machine learning models had negative predictive values of 94% for both outcomes.
Conclusions: Prediction models that incorporate longitudinal data can capture nonlinear disease progression in chronic hepatitis C and thus outperform baseline models. Machine learning methods can capture complex relationships between predictors and outcomes, yielding more accurate predictions; our models can help target costly therapies to patients with the most urgent need, guide the intensity of clinical monitoring required, and provide prognostic information to patients.
© 2015 by the American Association for the Study of Liver Diseases.
Figures







References
-
- Lawitz E, Mangia A, Wyles D, Rodriguez-Torres M, Hassanein T, Gordon SC, Schultz M, et al. Sofosbuvir for previously untreated chronic hepatitis C infection. N Engl J Med. 2013;368:1878–1887. - PubMed
-
- Therapeutics J. Olysio (simeprevir): Full prescribing information. 2013
-
- Medicine Io. Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C. 2010 Jan 11; 2010. - PubMed
-
- Moyer VA. Screening for hepatitis C virus infection in adults: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2013;159:349–357. - PubMed
-
- Smith BD, Morgan RL, Beckett GA, Falck-Ytter Y, Holtzman D, Teo CG, Jewett A, et al. Recommendations for the identification of chronic hepatitis C virus infection among persons born during 1945-1965. MMWR Recomm Rep. 2012;61:1–32. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
