Toward sophisticated basal ganglia neuromodulation: Review on basal ganglia deep brain stimulation
- PMID: 25684727
- PMCID: PMC4534367
- DOI: 10.1016/j.neubiorev.2015.02.003
Toward sophisticated basal ganglia neuromodulation: Review on basal ganglia deep brain stimulation
Abstract
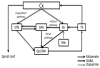
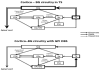
This review presents state-of-the-art knowledge about the roles of the basal ganglia (BG) in action-selection, cognition, and motivation, and how this knowledge has been used to improve deep brain stimulation (DBS) treatment of neurological and psychiatric disorders. Such pathological conditions include Parkinson's disease, Huntington's disease, Tourette syndrome, depression, and obsessive-compulsive disorder. The first section presents evidence supporting current hypotheses of how the cortico-BG circuitry works to select motor and emotional actions, and how defects in this circuitry can cause symptoms of the BG diseases. Emphasis is given to the role of striatal dopamine on motor performance, motivated behaviors and learning of procedural memories. Next, the use of cutting-edge electrochemical techniques in animal and human studies of BG functioning under normal and disease conditions is discussed. Finally, functional neuroimaging studies are reviewed; these works have shown the relationship between cortico-BG structures activated during DBS and improvement of disease symptoms.
Keywords: Deep brain stimulation; Electrochemistry; Functional magnetic resonance imaging; Globus pallidus; Human; Pig; Striatum; Substantia nigra; Subthalamic nucleus; Voltammetry.
Copyright © 2015 Elsevier Ltd. All rights reserved.
Figures




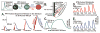


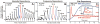



References
-
- Agnesi F, Tye SJ, Bledsoe JM, Griessenauer CJ, Kimble CJ, Sieck GC, Bennet KE, Garris PA, Blaha CD, Lee KH. Wireless Instantaneous Neurotransmitter Concentration System-based amperometric detection of dopamine, adenosine, and glutamate for intraoperative neurochemical monitoring. J Neurosurg. 2009;111:701–711. - PMC - PubMed
-
- Albin RL, Reiner A, Anderson KD, Penney JB, Young AB. Striatal and nigral neuron subpopulations in rigid Huntington’s disease - implications for the functional-anatomy of chorea and rigidity-akinesia. Ann Neurol. 1990;27:357–365. - PubMed
-
- Albin RL, Young AB, Penney JB. The functional-anatomy of basal ganglia disorders. Trends Neurosci. 1989;12:366–375. - PubMed
-
- Alderson HL, Latimer MP, Blaha CD, Phillips AG, Winn P. An examination of d-amphetamine self-administration in pedunculopontine tegmental nucleus-lesioned rats. Neuroscience. 2004;125:349–358. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

