Chronic hepatitis C genotype 1 treatment roadmap for resource constrained settings
- PMID: 25684966
- PMCID: PMC4323477
- DOI: 10.3748/wjg.v21.i6.1972
Chronic hepatitis C genotype 1 treatment roadmap for resource constrained settings
Abstract
Aim: To use existing hepatitis C virus (HCV) antiviral therapies as access to new treatments is limited.
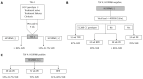
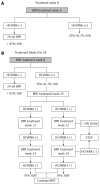
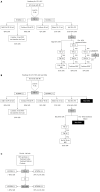
Methods: A PubMed search for randomised control trials or meta-analysis related to response-guided therapy of HCV genotype 1 patients was undertaken using pegylated interferon and ribavirin (PR), boceprevir (B) and telaprevir (T) and lead-in where response-guided therapy at TW4(TW4), 8(TW8), 10(TW10), or 12(TW12) based on HCVRNA(+) or HCVRNA(-). Studies presented at major conferences were also used. Where necessary, a post-hoc analysis was performed. A response-guided management roadmap was created based on sustained virological response (SVR).
Results: Starting with PR, those with HCVRNA(-) at TW4 have > 86% SVR, while those are HCVRNA(+) have 34%-41.7% SVR. HCVRNA(-) TW4 patients can have 24 wk PR if HCVRNA < 400000 IU/mL. Alternatively, 28 wk BPR has similar SVR. If HCVRNA(+) at TW4, 72 wk PR leads to 53% SVR, hence BPR is a better option, and if HCVRNA(-) by TW8, 28 wk therapy is sufficient. If HCVRNA(+) at TW8, then HCVRNA should be checked at TW10 and TW12. By TW12, HCVRNA ≥ 100 IU/mL activates the stopping rule. This roadmap is applicable for treatment-naïve, treatment failures and cirrhotic patients. Validation from an Asia Pacific early access boceprevir program confirmed the findings that HCVRNA(-) at TW4, or TW8 conferred > 80% SVR, leading to the "80-80" rule.
Conclusion: Using a roadmap based on HCVRNA(-) at TW4 or TW8 (the "80-80" rule), high SVR can be achieved, and guide the best choices for treatment, and also reduces drug exposure in poor responders.
Keywords: Boceprevir; Chronic hepatitis C; Cirrhosis; Partial responder; Peginterferon; Response-guided therapy; Ribavirin; Sustained virological response; Telaprevir; hepatitis C virus RNA.
Figures
References
-
- World Health Organization. WHO headquarters fact sheet: Hepatitis C. WHO Health Topics 2010. Available from: http://www.euro.who.int/en/health-topics/communicable-diseases/hepatitis....
-
- Casey LC, Lee WM. Hepatitis C virus therapy update 2013. Curr Opin Gastroenterol. 2013;29:243–249. - PubMed
-
- Food And Drug Administration. FDA approves new treatment for hepatitis C virus. 2013. Available from: http://www.fda.gov/newsevents/newsroom/pressannouncements/ucm376449.htm.
-
- Food And Drug Administration. Approval of Sovaldi (sofosbuvir) tablets for the treatment of chronic hepatitis C. 2013. Available from: http://www.fda.gov/forconsumers/byaudience/forpatientadvocates/ucm377920....
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

