Antioxidant polyphenol-rich extracts from the medicinal plants Antirhea borbonica, Doratoxylon apetalum and Gouania mauritiana protect 3T3-L1 preadipocytes against H2O2, TNFα and LPS inflammatory mediators by regulating the expression of superoxide dismutase and NF-κB genes
- PMID: 25685071
- PMCID: PMC4329220
- DOI: 10.1186/s12950-015-0055-6
Antioxidant polyphenol-rich extracts from the medicinal plants Antirhea borbonica, Doratoxylon apetalum and Gouania mauritiana protect 3T3-L1 preadipocytes against H2O2, TNFα and LPS inflammatory mediators by regulating the expression of superoxide dismutase and NF-κB genes
Abstract
Background: Adipose cells responsible for fat storage are the targets of reactive oxygen species (ROS) like H2O2 and pro-inflammatory agents including TNFα and LPS. Such mediators contribute to oxidative stress and alter inflammatory processes in adipose tissue, leading to insulin resistance during obesity. Thus, the identification of natural compounds such as plant polyphenols able to increase the antioxidant and anti-inflammatory capacity of the body is of high interest. We aimed to evaluate the biological properties of polyphenol-rich extracts from the medicinal plants A. borbonica, D. apetalum and G. mauritiana on preadipocytes exposed to H2O2, TNFα or LPS mediators.
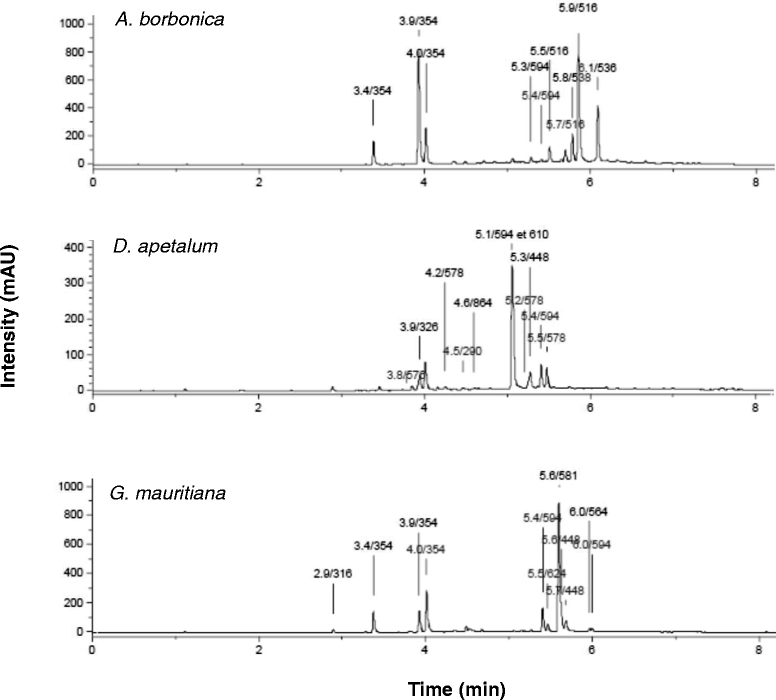
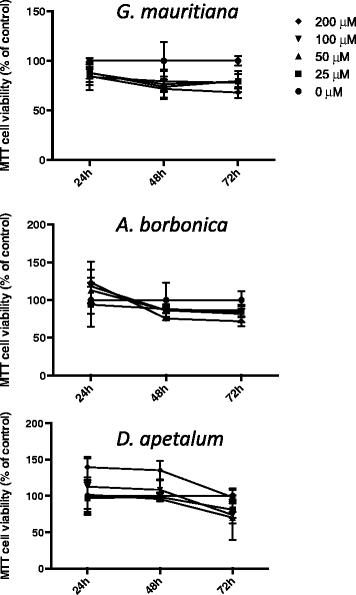
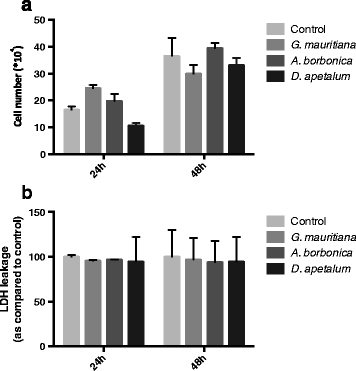
Methods: Medicinal plant extracts were analysed for their polyphenol contents by Folin-Ciocalteu and UPLC-ESI-MS methods as well as for their free radical-scavenging activities by DPPH and ORAC assays. To assess the ability of polyphenol-rich extracts to protect 3T3-L1 preadipocytes against H2O2, TNFα or LPS mediators, several parameters including cell viability (MTT and LDH assays), ROS production (DCFH-DA test), IL-6 and MCP-1 secretion (ELISA) were evaluated. Moreover, the expression of superoxide dismutase, catalase and NF-κB genes was explored (RT-QPCR).
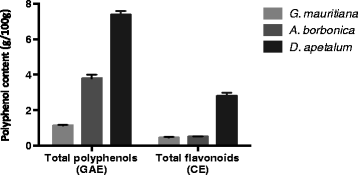
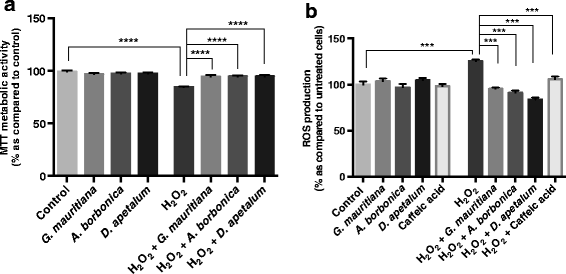
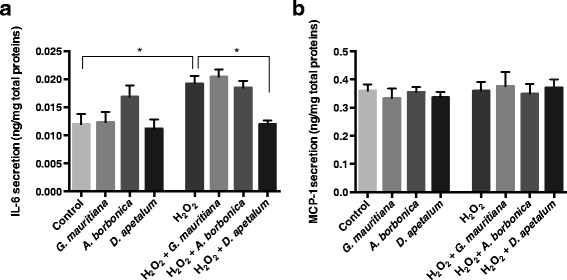
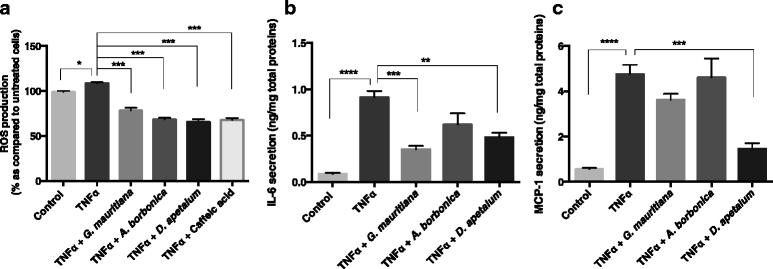
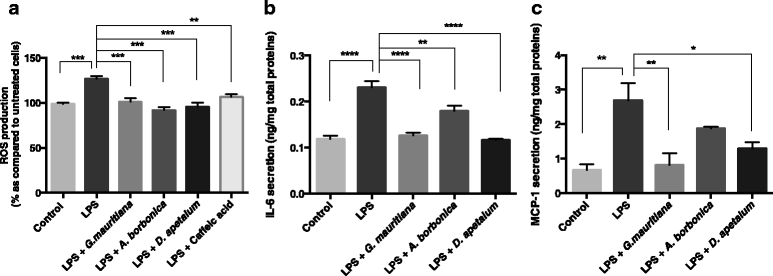
Results: All medicinal plants exhibited high levels of polyphenols with free radical-scavenging capacities. Flavonoids such as quercetin, kaempferol, epicatechin and procyanidins, and phenolic acids derived from caffeic acid including chlorogenic acid, were detected. Polyphenol-rich plant extracts did not exert a cytotoxic effect on preadipocytes but protected them against H2O2 anti-proliferative action. Importantly, they down-regulated ROS production and the secretion of IL-6 and MCP-1 pro-inflammatory markers induced by H2O2, TNFα and LPS mediators. Such a protective action was associated with an increase in superoxide dismutase antioxidant enzyme gene expression and a decrease in mRNA levels of NF-κB pro-inflammatory transcription factor.
Conclusion: This study highlights that antioxidant strategies based on polyphenols derived from medicinal plants tested could contribute to regulate adipose tissue redox status and immune process, and thus participate to the improvement of obesity-related oxidative stress and inflammation.
Keywords: Antioxidant strategies; Inflammation; Obesity; Oxidative stress; Plant polyphenols.
Figures


References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

