Targeted muscle reinnervation and advanced prosthetic arms
- PMID: 25685105
- PMCID: PMC4317279
- DOI: 10.1055/s-0035-1544166
Targeted muscle reinnervation and advanced prosthetic arms
Abstract
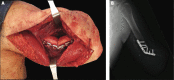
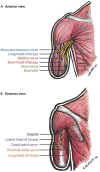
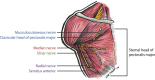
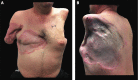
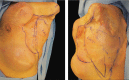
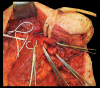
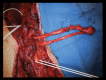
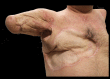
Targeted muscle reinnervation (TMR) is a surgical procedure used to improve the control of upper limb prostheses. Residual nerves from the amputated limb are transferred to reinnervate new muscle targets that have otherwise lost their function. These reinnervated muscles then serve as biological amplifiers of the amputated nerve motor signals, allowing for more intuitive control of advanced prosthetic arms. Here the authors provide a review of surgical techniques for TMR in patients with either transhumeral or shoulder disarticulation amputations. They also discuss how TMR may act synergistically with recent advances in prosthetic arm technologies to improve prosthesis controllability. Discussion of TMR and prosthesis control is presented in the context of a 41-year-old man with a left-side shoulder disarticulation and a right-side transhumeral amputation. This patient underwent bilateral TMR surgery and was fit with advanced pattern-recognition myoelectric prostheses.
Keywords: amputation; prosthetic limb; shoulder disarticulation; targeted muscle reinnervation; transhumeral amputation.
Figures
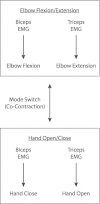

Similar articles
-
Targeted muscle reinnervation for improved control of myoelectric upper limb prostheses.J Biol Regul Homeost Agents. 2017 Oct-Dec;31(4 suppl 1):183-189. J Biol Regul Homeost Agents. 2017. PMID: 29188681
-
Targeted muscle reinnervation for real-time myoelectric control of multifunction artificial arms.JAMA. 2009 Feb 11;301(6):619-28. doi: 10.1001/jama.2009.116. JAMA. 2009. PMID: 19211469 Free PMC article.
-
Myoelectric prosthesis hand grasp control following targeted muscle reinnervation in individuals with transradial amputation.PLoS One. 2023 Jan 26;18(1):e0280210. doi: 10.1371/journal.pone.0280210. eCollection 2023. PLoS One. 2023. PMID: 36701412 Free PMC article.
-
Targeted Muscle Reinnervation and Regenerative Peripheral Nerve Interface for Myoelectric Prosthesis Control: The State of Evidence.Ann Plast Surg. 2025 Jun 1;94(6S Suppl 4):S572-S576. doi: 10.1097/SAP.0000000000004273. Ann Plast Surg. 2025. PMID: 40459463 Review.
-
Control Strategies and Performance Assessment of Upper-Limb TMR Prostheses: A Review.Sensors (Basel). 2021 Mar 10;21(6):1953. doi: 10.3390/s21061953. Sensors (Basel). 2021. PMID: 33802231 Free PMC article. Review.
Cited by
-
Long-term upper-extremity prosthetic control using regenerative peripheral nerve interfaces and implanted EMG electrodes.J Neural Eng. 2023 Apr 25;20(2):026039. doi: 10.1088/1741-2552/accb0c. J Neural Eng. 2023. PMID: 37023743 Free PMC article.
-
Physiotherapy: A potential and novel treatment approach for phantom limb pain in post-amputee patients - A systematic review.Br J Pain. 2024 Feb;18(1):5-27. doi: 10.1177/20494637231197002. Epub 2023 Aug 30. Br J Pain. 2024. PMID: 38344263 Free PMC article.
-
Clinical evaluation of the revolutionizing prosthetics modular prosthetic limb system for upper extremity amputees.Sci Rep. 2021 Jan 13;11(1):954. doi: 10.1038/s41598-020-79581-8. Sci Rep. 2021. PMID: 33441604 Free PMC article.
-
Targeted Muscle Reinnervation Compared to Standard Peripheral Nerve Management Following Amputation: A Systematic Review and Meta-Analysis.Hand (N Y). 2024 Oct 29:15589447241284811. doi: 10.1177/15589447241284811. Online ahead of print. Hand (N Y). 2024. PMID: 39469890 Free PMC article.
-
Innervation: the missing link for biofabricated tissues and organs.NPJ Regen Med. 2020 Jun 5;5:11. doi: 10.1038/s41536-020-0096-1. eCollection 2020. NPJ Regen Med. 2020. PMID: 32550009 Free PMC article. Review.
References
-
- Ziegler-Graham K, MacKenzie E J, Ephraim P L, Travison T G, Brookmeyer R. Estimating the prevalence of limb loss in the United States: 2005 to 2050. Arch Phys Med Rehabil. 2008;89(3):422–429. - PubMed
-
- Fischer H. Washington, DC: Congressional Research Service; 2010. US Military Casualty Statistics: Operation New Dawn, Operation Iraqi Freedom, and Operation Enduring Freedom; pp. 1–9.
-
- Sears H H. St Louis, MO: Mosby; 1992. Trends in upper-extremity prosthetics development; pp. 345–356.
-
- Jensen T S, Krebs B, Nielsen J, Rasmussen P. Phantom limb, phantom pain and stump pain in amputees during the first 6 months following limb amputation. Pain. 1983;17(3):243–256. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

