Palladium-Catalyzed Direct α-Aryation of Benzyl Thioethers with Aryl Bromides
- PMID: 25685127
- PMCID: PMC4323356
- DOI: 10.1002/adsc.201400679
Palladium-Catalyzed Direct α-Aryation of Benzyl Thioethers with Aryl Bromides
Abstract
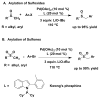
The arylation of sp3-hybridized C-H's bonds is a powerful strategy to build molecular complexity and diversity. A novel and efficient palladium-catalyzed direct sp3 C-H arylation of aryl and alkyl benzyl thioether derivatives with aryl bromides is reported. The reaction involves reversible deprotonation of the benzylic C-H's of the thioether with either LiN(SiMe3)2 or NaN(SiMe3)2 and subsequent cross-coupling to provide the functionalized products in up to 97% yield. A screen of 24 of the most successful ligands in cross-coupling chemistry led to the identification of NiXantPhos as the only viable ligand for this challenging coupling.
Keywords: Arylation; Cross-Coupling; C–H functionalization; Palladium; Sulfides.
Figures



References
-
- Baudoin O. Chem Soc Rev. 2011;40:4902–4911. - PubMed
- Bellina F, Rossi R. Chem Rev. 2010;110:1082–1146. - PubMed
- Daugulis O, Do HQ, Shabashov D. Acc Chem Res. 2009;42:1074–1086. - PMC - PubMed
- Engle KM, Mei TS, Wasa M, Yu JQ. Acc Chem Res. 2011;45:788–802. - PMC - PubMed
- Lyons TW, Sanford MS. Chem Rev. 2010;110:1147–1169. - PMC - PubMed
- Colby DA, Bergman RG, Ellman JA. Chem Rev. 2010;110:624–655. - PMC - PubMed
- Kakiuchi F, Kochi T. Synthesis. 2008;2008:3013–3039.
- Alberico D, Scott ME, Lautens M. Chem Rev. 2007;107:174–238. - PubMed
- Ackermann L. Chem Rev. 2011;111:1315–1345. - PubMed
- Rubin M, Rubina M, Gevorgyan V. Chem Rev. 2007;107:3117–3179. - PubMed
- Wencel-Delord J, Droge T, Liu F, Glorius F. Chem Soc Rev. 2011;40:4740–4761. - PubMed
- Wencel-Delord J, Glorius F. Nat Chem. 2013;5:369–375. - PubMed
- Mkhalid IAI, Barnard JH, Marder TB, Murphy JM, Hartwig JF. Chem Rev. 2010;110:890–931. - PubMed
- Campeau LC, Fagnou K. Chem Soc Rev. 2007;36:1058–1068. - PubMed
-
- McGrew GI, Temaismithi J, Carroll PJ, Walsh PJ. Angew Chem, Int Ed. 2010;49:5541–5544. - PMC - PubMed
- Zhang J, Stanciu C, Wang B, Hussain MM, Da CS, Carroll PJ, Dreher SD, Walsh PJ. J Am Chem Soc. 2011;133:20552–20560. - PMC - PubMed
- McGrew GI, Stanciu C, Zhang J, Carroll PJ, Dreher SD, Walsh PJ. Angew Chem, Int Ed. 2012;51:11510–11513. - PubMed
- Sha SC, Zhang J, Carroll PJ, Walsh PJ. J Am Chem Soc. 2013;135:17602–17609. - PMC - PubMed
-
- Zhang J, Bellomo A, Creamer AD, Dreher SD, Walsh PJ. J Am Chem Soc. 2012;134:13765–13772. - PubMed
-
- Bellomo A, Zhang J, Trongsiriwat N, Walsh PJ. Chem Sci. 2013;4:849–857.
-
- Cremlyn RJ. An Introduction to Organosulfur Chemistry. Wiley; 1996.
- Top 200 Prescription Drugs of 2009. 2010 avaiable at http://www.pharmacytimes.com/publications/issue/2010/may2010/rxfocustopd....
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
