Mesenchymal Stem Cells Pretreated with HGF and FGF4 Can Reduce Liver Fibrosis in Mice
- PMID: 25685159
- PMCID: PMC4320872
- DOI: 10.1155/2015/747245
Mesenchymal Stem Cells Pretreated with HGF and FGF4 Can Reduce Liver Fibrosis in Mice
Abstract
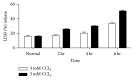
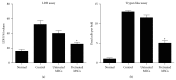
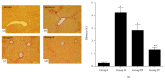
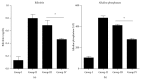
Stem cells have opened a new avenue to treat liver fibrosis. We investigated in vitro and in vivo the effect of cytokine (HGF and FGF4) pretreated MSCs in reduction of CCl4 liver injury. Mouse MSCs were pretreated with cytokines to improve their ability to reduce CCl4 injury. In vitro we gave CCl4 injury to mouse hepatocytes and cocultured it with untreated and cytokines pretreated MSCs. For in vivo study we labeled MSCs with PKH-26 and transplanted them into CCl4 injured mice by direct injection into liver. In vitro data showed that cytokines pretreated MSCs significantly reduce LDH level and apoptotic markers in CCl4 injured hepatocytes cocultured model. Furthermore the cytokines pretreated MSCs also improved cell viability and enhanced hepatic and antiapoptotic markers in injured hepatocytes cocultured model as compared to untreated MSCs. In vivo data in cytokines pretreated group demonstrated greater homing of MSCs in liver, restored glycogen storage, and significant reduction in collagen, alkaline phosphatase, and bilirubin levels. TUNEL assay and real time PCR also supported our hypothesis. Therefore, cytokines pretreated MSCs were shown to have a better therapeutic potential on reduction of liver injury. These results demonstrated the potential utility of this novel idea of cytokines pretreated MSCs for the treatment of liver fibrosis.
Figures







References
-
- Friedman S. L. Mechanisms of disease: mechanisms of hepatic fibrosis and therapeutic implications. Nature Clinical Practice: Gastroenterology & Hepatology. 2004;1(2):98–105. - PubMed
-
- Rabani V., Shahsavani M., Gharavi M., Piryaei A., Azhdari Z., Baharvand H. Mesenchymal stem cell infusion therapy in a carbon tetrachloride-induced liver fibrosis model affects matrix metalloproteinase expression. Cell Biology International. 2010;34(6):601–605. doi: 10.1042/CBI20090386. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

