The efficacy and safety of the Timothy grass allergy sublingual immunotherapy tablet in Canadian adults and children
- PMID: 25685162
- PMCID: PMC4326370
- DOI: 10.1186/1710-1492-10-53
The efficacy and safety of the Timothy grass allergy sublingual immunotherapy tablet in Canadian adults and children
Abstract
Background: The effect of sublingual Timothy grass immunotherapy tablet 2800 BAU (grass SLIT-T) has been evaluated in three North American trials in adults and children who have allergic rhinitis with or without conjunctivitis (AR/C). This paper examines the effects of grass SLIT-T in Canadians.
Methods: Data for grass-allergic Canadians in three randomized, placebo-controlled, double-blind trials were analyzed post hoc: 1) adults ≥18 y, grass-pollen season [GPS] 2009; 2) children 5- <18 y, 2009; and 3) adults 18-65 y and children 5- <18 y, GPS 2012. Data from the GPS 2009 trials were pooled to provide a more precise estimate of treatment effects than the individual studies would provide. In every trial, participants received once-daily grass SLIT-T or placebo approximately 12 weeks before and continuing throughout the GPS. Participants used daily electronic diaries to record AR/C symptoms and medication use for treatment of symptoms. The therapeutic effect of grass SLIT-T was measured as a total combined score (TCS = daily symptom score + daily medication score) averaged over the entire GPS. Safety was assessed by monitoring adverse events (AEs).
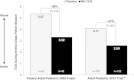
Results: In the three trials, 386 Canadian participants were randomized; the overall population had 2284 participants. Canadian participants treated with grass SLIT-T in the pooled adult-pediatric 2009 trials showed a 38% mean TCS reduction relative to placebo (-2.06 difference [95% CI: -3.72, -0.39]; 3.32 vs. 5.37). Participants treated with grass SLIT-T in the adult-pediatric 2012 trial showed a 37% median TCS reduction relative to placebo (-1.53 difference [95% CI: -2.1, -0.3]; 2.58 vs. 4.11). Similar efficacy findings were observed over the peak GPS. Approximately 90% of treatment-related AEs were mild or moderate in severity. Two Canadian participants had moderate systemic allergic reactions (skin, respiratory, abdominal symptoms) to grass SLIT-T; symptoms resolved within 1 hour without medical intervention or treatment. No serious or life-threatening treatment-related AEs occurred.
Conclusion: The 2800 BAU Timothy grass SLIT-T significantly improved AR/C induced by Timothy grass pollen in adults and children ≥5 y in Canadians, which was consistent with the robust efficacy observed in the overall trial population. The treatment was generally well tolerated.
Trial registration: Clinicaltrials.gov identifiers NCT00562159, NCT00550550, NCT01385371.
Keywords: Allergic rhinitis; Conjunctivitis; Sublingual immunotherapy tablet; Timothy grass pollen.
Figures

References
-
- Allergies & Asthma. [http://www.allergyfoundation.ca/website/asthma_allergies_brochure.pdf]
-
- Aït-Khaled N, Pearce N, Anderson HR, Ellwood P, Montefort S, Shah J, ISAAC Phase Three Study Group Global map of the prevalence of symptoms of rhinoconjunctivitis in children: The International Study of Asthma and Allergies in Childhood (ISAAC) Phase Three. Allergy. 2009;64(1):123–148. doi: 10.1111/j.1398-9995.2008.01884.x. - DOI - PubMed
-
- Climate Change and Public Health Factsheets. Public Health Agency of Canada. [http://www.phac-aspc.gc.ca/hp-ps/eph-esp/fs-fi-b-eng.php]
-
- Chan-Yeung M, Anthonisen NR, Becklake MR, Bowie D, Sonia Buist A, Dimich-Ward H, Ernst P, Sears MR, Siersted HC, Sweet L, Van Til L, Manfreda J. Geographical variations in the prevalence of atopic sensitization in six study sites across Canada. Allergy. 2010;65(11):1404–1413. doi: 10.1111/j.1398-9995.2010.02399.x. - DOI - PubMed
-
- Martin BG, Mansfield LE, Nelson HS. Cross-allergenicity among the grasses. Ann Allergy. 1985;54(2):99–104. - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

