Cardiomyocyte glucagon receptor signaling modulates outcomes in mice with experimental myocardial infarction
- PMID: 25685700
- PMCID: PMC4314543
- DOI: 10.1016/j.molmet.2014.11.005
Cardiomyocyte glucagon receptor signaling modulates outcomes in mice with experimental myocardial infarction
Abstract
Objective: Glucagon is a hormone with metabolic actions that maintains normoglycemia during the fasting state. Strategies enabling either inhibition or activation of glucagon receptor (Gcgr) signaling are being explored for the treatment of diabetes or obesity. However, the cardiovascular consequences of manipulating glucagon action are poorly understood.
Methods: We assessed infarct size and the following outcomes following left anterior descending (LAD) coronary artery ligation; cardiac gene and protein expression, acylcarnitine profiles, and cardiomyocyte survival in normoglycemic non-obese wildtype mice, and in newly generated mice with selective inactivation of the cardiomyocyte Gcgr. Complementary experiments analyzed Gcgr signaling and cell survival in cardiomyocyte cultures and cell lines, in the presence or absence of exogenous glucagon.
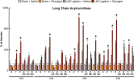
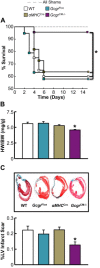
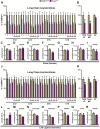
Results: Exogenous glucagon administration directly impaired recovery of ventricular pressure in ischemic mouse hearts ex vivo, and increased mortality from myocardial infarction after LAD coronary artery ligation in mice in a p38 MAPK-dependent manner. In contrast, cardiomyocyte-specific reduction of glucagon action in adult Gcgr (CM-/-) mice significantly improved survival, and reduced hypertrophy and infarct size following myocardial infarction. Metabolic profiling of hearts from Gcgr (CM-/-) mice revealed a marked reduction in long chain acylcarnitines in both aerobic and ischemic hearts, and following high fat feeding, consistent with an essential role for Gcgr signaling in the control of cardiac fatty acid utilization.
Conclusions: Activation or reduction of cardiac Gcgr signaling in the ischemic heart produces substantial cardiac phenotypes, findings with implications for therapeutic strategies designed to augment or inhibit Gcgr signaling for the treatment of metabolic disorders.
Keywords: Cardiomyocytes; Diabetes; Fatty acid metabolism; Glucagon; Glucagon receptor; Heart; Myocardial infarction.
Figures




References
-
- Sinclair E.M., Yusta B., Streutker C., Baggio L.L., Koehler J., Charron M.J. Glucagon receptor signaling is essential for control of murine hepatocyte survival. Gastroenterology. 2008;135(6):2096–2106. - PubMed
-
- Dunphy J.L., Taylor R.G., Fuller P.J. Tissue distribution of rat glucagon receptor and GLP-1 receptor gene expression. Molecular and Cellular Endocrinology. 1998;141(1–2):179–186. - PubMed
-
- Campos R.V., Lee Y.C., Drucker D.J. Divergent tissue-specific and developmental expression of receptors for glucagon and glucagon-like peptide-1 in the mouse. Endocrinology. 1994;134:2156–2164. - PubMed
-
- Ali S., Drucker D.J. Benefits and limitations of reducing glucagon action for the treatment of type 2 diabetes. American Journal of Physiology. Endocrinology and Metabolism. 2009;296(3):E415–E421. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

