Has Stewart approach improved our ability to diagnose acid-base disorders in critically ill patients?
- PMID: 25685724
- PMCID: PMC4326765
- DOI: 10.5492/wjccm.v4.i1.62
Has Stewart approach improved our ability to diagnose acid-base disorders in critically ill patients?
Abstract
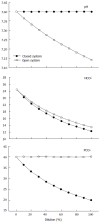
The Stewart approach-the application of basic physical-chemical principles of aqueous solutions to blood-is an appealing method for analyzing acid-base disorders. These principles mainly dictate that pH is determined by three independent variables, which change primarily and independently of one other. In blood plasma in vivo these variables are: (1) the PCO2; (2) the strong ion difference (SID)-the difference between the sums of all the strong (i.e., fully dissociated, chemically nonreacting) cations and all the strong anions; and (3) the nonvolatile weak acids (Atot). Accordingly, the pH and the bicarbonate levels (dependent variables) are only altered when one or more of the independent variables change. Moreover, the source of H(+) is the dissociation of water to maintain electroneutrality when the independent variables are modified. The basic principles of the Stewart approach in blood, however, have been challenged in different ways. First, the presumed independent variables are actually interdependent as occurs in situations such as: (1) the Hamburger effect (a chloride shift when CO2 is added to venous blood from the tissues); (2) the loss of Donnan equilibrium (a chloride shift from the interstitium to the intravascular compartment to balance the decrease of Atot secondary to capillary leak; and (3) the compensatory response to a primary disturbance in either independent variable. Second, the concept of water dissociation in response to changes in SID is controversial and lacks experimental evidence. In addition, the Stewart approach is not better than the conventional method for understanding acid-base disorders such as hyperchloremic metabolic acidosis secondary to a chloride-rich-fluid load. Finally, several attempts were performed to demonstrate the clinical superiority of the Stewart approach. These studies, however, have severe methodological drawbacks. In contrast, the largest study on this issue indicated the interchangeability of the Stewart and conventional methods. Although the introduction of the Stewart approach was a new insight into acid-base physiology, the method has not significantly improved our ability to understand, diagnose, and treat acid-base alterations in critically ill patients.
Keywords: Acid-base metabolism; Anion gap; Base excess; Bicarbonate; Stewart approach; Strong ion difference; Strong ion gap.
Figures




Similar articles
-
[What is the contribution of Stewart's concept in acid-base disorders analysis?].Ann Fr Anesth Reanim. 2007 May;26(5):423-33. doi: 10.1016/j.annfar.2007.02.012. Epub 2007 Apr 25. Ann Fr Anesth Reanim. 2007. PMID: 17462852 Review. French.
-
[Clinical evaluation of acid-base status: Henderson-Hasselbalch, or Stewart-Fencl approach?].Cas Lek Cesk. 2016 Winter;155(7):365-369. Cas Lek Cesk. 2016. PMID: 27990831 Review. Czech.
-
[The Stewart model. "Modern" approach to the interpretation of the acid-base metabolism].Anaesthesist. 2004 Apr;53(4):347-57. doi: 10.1007/s00101-004-0660-x. Anaesthesist. 2004. PMID: 15088097 Review. German.
-
[Does Stewart-Fencl improve the evaluation of acid-base status in stable patients on hemodiafiltration?].Nefrologia. 2010;30(2):214-9. doi: 10.3265/Nefrologia.pre2009.Dic.5774. Epub 2009 Dec 14. Nefrologia. 2010. PMID: 20038966 Spanish.
-
[Analysis and adjustment of acid-base disturbances according to the Stewart-Fencl principle].Vnitr Lek. 2004 Jul;50(7):526-30. Vnitr Lek. 2004. PMID: 15323260 Czech.
Cited by
-
Acid-Base Disorders in the Critically Ill Patient.Clin J Am Soc Nephrol. 2023 Jan 1;18(1):102-112. doi: 10.2215/CJN.04500422. Epub 2022 Aug 23. Clin J Am Soc Nephrol. 2023. PMID: 35998977 Free PMC article.
-
Acid-base disturbances in nephrotic syndrome: analysis using the CO2/HCO3 method (traditional Boston model) and the physicochemical method (Stewart model).Clin Exp Nephrol. 2017 Oct;21(5):866-876. doi: 10.1007/s10157-017-1387-8. Epub 2017 Mar 13. Clin Exp Nephrol. 2017. PMID: 28289910 Free PMC article.
-
Effect of Intravenously Administered Crystalloid Solutions on Acid-Base Balance in Domestic Animals.J Vet Intern Med. 2017 Sep;31(5):1371-1381. doi: 10.1111/jvim.14803. Epub 2017 Aug 20. J Vet Intern Med. 2017. PMID: 28833697 Free PMC article. Review.
-
Serum chloride levels in critical illness-the hidden story.Intensive Care Med Exp. 2018 Apr 13;6(1):10. doi: 10.1186/s40635-018-0174-5. Intensive Care Med Exp. 2018. PMID: 29654387 Free PMC article. Review.
-
Impact of chloride and strong ion difference on ICU and hospital mortality in a mixed intensive care population.Ann Intensive Care. 2016 Dec;6(1):91. doi: 10.1186/s13613-016-0193-x. Epub 2016 Sep 17. Ann Intensive Care. 2016. PMID: 27639981 Free PMC article.
References
-
- Stewart PA. Modern quantitative acid-base chemistry. Can J Physiol Pharmacol. 1983;61:1444–1461. - PubMed
-
- Siggaard-Andersen O, Fogh-Andersen N. Base excess or buffer base (strong ion difference) as measure of a non-respiratory acid-base disturbance. Acta Anaesthesiol Scand Suppl. 1995;107:123–128. - PubMed
-
- Narins RG. (Editor). Maxwell and Kleeman’s Clinical Disorders of Fluid and Electrolyte Metabolism. 5th ed. New York: McGraw-Hill; 1994.
-
- Narins RG, Emmett M. Simple and mixed acid-base disorders: a practical approach. Medicine (Baltimore) 1980;59:161–187. - PubMed
-
- Siggaard-Andersen O. The Acid-Base Status of the Blood. 4th ed. Baltimore: Williams & Wilkins; 1974.
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

