Equine adipose-derived mesenchymal stem cells: phenotype and growth characteristics, gene expression profile and differentiation potentials
- PMID: 25685736
- PMCID: PMC4297484
- DOI: 10.22074/cellj.2015.491
Equine adipose-derived mesenchymal stem cells: phenotype and growth characteristics, gene expression profile and differentiation potentials
Abstract
Objective: Because of the therapeutic application of stem cells (SCs), isolation and characterization of different types of SCs, especially mesenchymal stem cells (MSCs), have gained considerable attention in recent studies. Adipose tissue is an abundant and accessible source of MSCs which can be used for tissue engineering and in particular for treatment of musculoskeletal disorders. This study was aimed to isolate and culture equine adipose-derived MSCs (AT-MSCs) from little amounts of fat tissue samples and determine some of their biological characteristics.
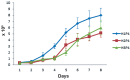
Materials and methods: In this descriptive study, only 3-5 grams of fat tissue were collected from three crossbred mares. Immediately, cells were isolated by mechanical means and enzymatic digestion and were cultured in optimized conditions until passage 3 (P3). The cells at P3 were evaluated for proliferative capacities, expression of specific markers, and osteogenic, chondrogenic and adipogenic differentiation potentials.
Results: Results showed that the isolated cells were plastic adherent with a fibroblast-like phenotype. AT-MSCs exhibited expression of mesenchymal cluster of differentiation (CD) markers (CD29, CD44 and CD90) and not major histocompatibility complex II (MHC-II) and CD34 (hematopoietic marker). Cellular differentiation assays demonstrated the chondrogenic, adipogenic and osteogenic potential of the isolated cells.
Conclusion: Taken together, our findings reveal that equine MSCs can be obtained easily from little amounts of fat tissue which can be used in the future for regenerative purposes in veterinary medicine.
Keywords: Adipose; Characterization; Differentiation; Equine; Mesenchymal Stem Cells.
Figures




References
-
- Guest DJ, Smith MR, Allen WR. Monitoring the fate of autologous and allogeneic mesenchymal progenitor cells injected into the superficial digital flexor tendon of horses: preliminary study. Equine Vet J. 2008;40(2):178–181. - PubMed
-
- Nixon AJ, Dahlgren LA, Haupt JL, Yeager AE, Ward DL. Effect of adipose-derived nucleated cell fractions on tendon repair in horses with collagenase-induced tendinitis. Am J Vet Res. 2008;69(7):928–937. - PubMed
-
- Smith RK, Korda M, Blunn GW, Goodship AE. Isolation and implantation of autologous equine mesenchymal stem cells from bone marrow into the superficial digital flexor tendon as a potential novel treatment. Equine Vet J. 2003;35(1):99–102. - PubMed
-
- Ribitsch I, Burk J, Delling U, Geißler C, Gittel C, Julke H, et al. Basic science and clinical application of stem cells in veterinary medicine. Adv Biochem Eng Biotechnol. 2010;123:219–263. - PubMed
-
- English K, French A, Wood KJ. Mesenchymal stromal cells: facilitators of successful transplantation? Cell Stem Cell. 2010;7(4):431–442. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
