Fabrication and characterization of spongy denuded amniotic membrane based scaffold for tissue engineering
- PMID: 25685738
- PMCID: PMC4297486
- DOI: 10.22074/cellj.2015.493
Fabrication and characterization of spongy denuded amniotic membrane based scaffold for tissue engineering
Abstract
Objective: As a biological tissue material, amniotic membrane (AM) has low immunogenicity and to date has been widely adopted in clinical practice. However, some features such as low biomechanical consistency and rapid biodegradation is limited the application of AM. Therefore, in this study, we fabricated a novel three-dimensional (3D) spongy scaffold made of the extracellular matrix (ECM) of denuded AM. Due to their unique characteristics which are similar to the skin, these scaffolds can be considered as an alternative option in skin tissue engineering.
Materials and methods: In this experimental study, cellular components of human amniotic membrane (HAM) were removed with 0.03% (w/v) sodium dodecyl sulphate (SDS). Quantitative analysis was performed to determine levels of Glycosaminoglycans (GAGs), collagen, and deoxyribonucleic acid (DNA). To increase the low efficiency and purity of the ECM component, especially collagen and GAG, we applied an acid solubilization procedure hydrochloridric acid (HCl 0.1 M) with pepsin (1 mg/ml). In the present experiment 1-ethyl-3-(3-dimethyl aminopropyl) carbodiimide hydrochloride (EDC)/N-hydroxysuccinimide (NHS) cross linker agent was used to improve the mechanical properties of 3D lyophilized AM scaffold. The spongy 3D AM scaffolds were specified, by scanning electron microscopy, hematoxylin and eosin (H&E) staining, a swelling test, and mechanical strength and in vitro biodegradation tests. Human fetal fibroblast culture systems were used to establish that the scaffolds were cytocompatible.
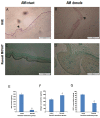
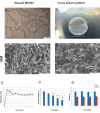
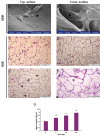
Results: Histological analysis of treated human AM showed impressive removal of cellular components. DNA content was diminished after treatment (39 ± 4.06 μg/ml vs. 341 ± 29.60 μg/ml). Differences were observed between cellular and denude AM in matrix collagen (478 ± 18.06 μg/mg vs. 361 ± 27.47 μg/mg).With the optimum concentration of 1 mM NHS/EDC ratio1:4, chemical cross-linker agent could significantly increase the mechanical property, and resistance to collagenase digestion. The results of 2, 4, 6-Trinitrobenzenesulfonic acid (TNBS) test showed that cross-linking efficiency of AM derived ECM scaffolds was about 65% ± 10.53. Scaffolds treated with NHS/EDC cross-linker agent by 100 μg/ml collagenase, lost 75% of their dry weight after 14 days. The average pore size of 3D spongy scaffold was 160 µm measured from scanning electron microscope (SEM) images that it is suitable for cell penetration, nutrients and gas change. In addition, the NHS/ EDC cross-linked AM scaffolds were able to support human fetal fibroblast cell proliferation in vitro. Extracts and contact prepared from the 3D spongy scaffold of AM showed a significant increase in the attachment and proliferation of the human fetal fibroblasts cells.
Conclusion: The extra-cellular matrix of denuded AM-based scaffold displays the main properties required for substitute skin including natural in vitro biodegradation, similar physical and mechanical characterization, nontoxic biomaterial and no toxic effect on cell attachment and cell proliferation.
Keywords: Biodegradable; Extracellular Matrix; Skin Substitute.
Figures
References
-
- van der Veen VC, van der Wal MB, van Leeuwen MC, Ulrich MM, Middelkoop E. Biological background of dermal substitutes. Burns. 2010;36(3):305–321. - PubMed
-
- Uijtdewilligen PJ, Versteeg EM, Gilissen C, van Reijmersdal SV, Schoppmeyer R, Wismans RG, et al. Towards embryonic-like scaffolds for skin tissue engineering: identification of effector molecules and construction of scaffolds. J Tissue Eng Regen Med. 2013 (In Press) - PubMed
-
- Duan X, Sheardown H. Crosslinking of collagen with dendrimers. J Biomed Mater Res A. 2005;75(3):510–518. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
