Prokineticin 2 upregulation in the peripheral nervous system has a major role in triggering and maintaining neuropathic pain in the chronic constriction injury model
- PMID: 25685780
- PMCID: PMC4313068
- DOI: 10.1155/2015/301292
Prokineticin 2 upregulation in the peripheral nervous system has a major role in triggering and maintaining neuropathic pain in the chronic constriction injury model
Abstract
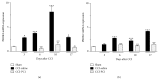
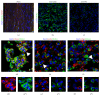
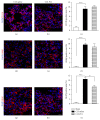
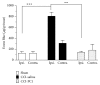
The new chemokine Prokineticin 2 (PROK2) and its receptors (PKR1 and PKR2) have a role in inflammatory pain and immunomodulation. Here we identified PROK2 as a critical mediator of neuropathic pain in the chronic constriction injury (CCI) of the sciatic nerve in mice and demonstrated that blocking the prokineticin receptors with two PKR1-preferring antagonists (PC1 and PC7) reduces pain and nerve damage. PROK2 mRNA expression was upregulated in the injured nerve since day 3 post injury (dpi) and in the ipsilateral DRG since 6 dpi. PROK2 protein overexpression was evident in Schwann Cells, infiltrating macrophages and axons in the peripheral nerve and in the neuronal bodies and some satellite cells in the DRG. Therapeutic treatment of neuropathic mice with the PKR-antagonist, PC1, impaired the PROK2 upregulation and signalling. This fact, besides alleviating pain, brought down the burden of proinflammatory cytokines in the damaged nerve and prompted an anti-inflammatory repair program. Such a treatment also reduced intraneural oedema and axon degeneration as demonstrated by the physiological skin innervation and thickness conserved in CCI-PC1 mice. These findings suggest that PROK2 plays a crucial role in neuropathic pain and might represent a novel target of treatment for this disease.
Figures








Similar articles
-
Controlling the activation of the Bv8/prokineticin system reduces neuroinflammation and abolishes thermal and tactile hyperalgesia in neuropathic animals.Br J Pharmacol. 2014 Nov;171(21):4850-65. doi: 10.1111/bph.12793. Epub 2014 Sep 5. Br J Pharmacol. 2014. PMID: 24902717 Free PMC article.
-
PC1, a non-peptide PKR1-preferring antagonist, reduces pain behavior and spinal neuronal sensitization in neuropathic mice.Pharmacol Res. 2015 Jan;91:36-46. doi: 10.1016/j.phrs.2014.11.004. Epub 2014 Nov 27. Pharmacol Res. 2015. PMID: 25434589
-
Increased prokineticin 2 expression in gut inflammation: role in visceral pain and intestinal ion transport.Neurogastroenterol Motil. 2012 Jan;24(1):65-75, e12. doi: 10.1111/j.1365-2982.2011.01804.x. Epub 2011 Nov 3. Neurogastroenterol Motil. 2012. PMID: 22050240
-
Targeting the Prokineticin System to Control Chronic Pain and Inflammation.Curr Med Chem. 2018;25(32):3883-3894. doi: 10.2174/0929867324666170713102514. Curr Med Chem. 2018. PMID: 28707588 Review.
-
Bv8/PK2 and prokineticin receptors: a druggable pronociceptive system.Curr Opin Pharmacol. 2012 Feb;12(1):62-6. doi: 10.1016/j.coph.2011.10.023. Epub 2011 Nov 30. Curr Opin Pharmacol. 2012. PMID: 22136937 Review.
Cited by
-
Prokineticin Receptor Inhibition With PC1 Protects Mouse Primary Sensory Neurons From Neurotoxic Effects of Chemotherapeutic Drugs in vitro.Front Immunol. 2020 Sep 24;11:2119. doi: 10.3389/fimmu.2020.02119. eCollection 2020. Front Immunol. 2020. PMID: 33072073 Free PMC article.
-
Targeting prokineticin system counteracts hypersensitivity, neuroinflammation, and tissue damage in a mouse model of bortezomib-induced peripheral neuropathy.J Neuroinflammation. 2019 Apr 17;16(1):89. doi: 10.1186/s12974-019-1461-0. J Neuroinflammation. 2019. PMID: 30995914 Free PMC article.
-
Optimal Delivery of Pain Management in Schwannomatosis: A Literature Review.Ther Clin Risk Manag. 2025 Jan 17;21:61-68. doi: 10.2147/TCRM.S362794. eCollection 2025. Ther Clin Risk Manag. 2025. PMID: 39839825 Free PMC article. Review.
-
Prokineticin 2 is a catabolic regulator of osteoarthritic cartilage destruction in mouse.Arthritis Res Ther. 2023 Dec 6;25(1):236. doi: 10.1186/s13075-023-03206-4. Arthritis Res Ther. 2023. PMID: 38057865 Free PMC article.
-
Transcriptome sequencing and multi-plex imaging of prostate cancer microenvironment reveals a dominant role for monocytic cells in progression.BMC Cancer. 2021 Jul 22;21(1):846. doi: 10.1186/s12885-021-08529-6. BMC Cancer. 2021. PMID: 34294073 Free PMC article.
References
-
- Giannini E., Lattanzi R., Nicotra A., et al. The chemokine Bv8/prokineticin 2 is up-regulated in inflammatory granulocytes and modulates inflammatory pain. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(34):14646–14651. doi: 10.1073/pnas.0903720106. - DOI - PMC - PubMed
-
- LeCouter J., Zlot C., Tejada M., Peale F., Ferrara N. Bv8 and endocrine gland-derived vascular endothelial growth factor stimulate hematopoiesis and hematopoietic cell mobilization. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(48):16813–16818. doi: 10.1073/pnas.0407697101. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

