Mechanical stress promotes cisplatin-induced hepatocellular carcinoma cell death
- PMID: 25685789
- PMCID: PMC4317602
- DOI: 10.1155/2015/430569
Mechanical stress promotes cisplatin-induced hepatocellular carcinoma cell death
Abstract
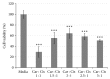
Cisplatin (CisPt) is a commonly used platinum-based chemotherapeutic agent. Its efficacy is limited due to drug resistance and multiple side effects, thereby warranting a new approach to improving the pharmacological effect of CisPt. A newly developed mathematical hypothesis suggested that mechanical loading, when coupled with a chemotherapeutic drug such as CisPt and immune cells, would boost tumor cell death. The current study investigated the aforementioned mathematical hypothesis by exposing human hepatocellular liver carcinoma (HepG2) cells to CisPt, peripheral blood mononuclear cells, and mechanical stress individually and in combination. HepG2 cells were also treated with a mixture of CisPt and carnosine with and without mechanical stress to examine one possible mechanism employed by mechanical stress to enhance CisPt effects. Carnosine is a dipeptide that reportedly sequesters platinum-based drugs away from their pharmacological target-site. Mechanical stress was achieved using an orbital shaker that produced 300 rpm with a horizontal circular motion. Our results demonstrated that mechanical stress promoted CisPt-induced death of HepG2 cells (~35% more cell death). Moreover, results showed that CisPt-induced death was compromised when CisPt was left to mix with carnosine 24 hours preceding treatment. Mechanical stress, however, ameliorated cell death (20% more cell death).
Figures






Similar articles
-
Evaluation of Cisplatin Efficacy on HepG2 and E. coli Cells under Acidic Conditions.Asian Pac J Cancer Prev. 2019 Mar 26;20(3):723-726. doi: 10.31557/APJCP.2019.20.3.723. Asian Pac J Cancer Prev. 2019. PMID: 30909670 Free PMC article.
-
VCC-1 over-expression inhibits cisplatin-induced apoptosis in HepG2 cells.Biochem Biophys Res Commun. 2012 Apr 6;420(2):336-42. doi: 10.1016/j.bbrc.2012.02.160. Epub 2012 Mar 7. Biochem Biophys Res Commun. 2012. PMID: 22425983
-
Inhibition of the JNK signaling pathway increases sensitivity of hepatocellular carcinoma cells to cisplatin by down-regulating expression of P-glycoprotein.Eur Rev Med Pharmacol Sci. 2016;20(6):1098-108. Eur Rev Med Pharmacol Sci. 2016. PMID: 27049263
-
P-glycoprotein depresses cisplatin sensitivity in L1210 cells by inhibiting cisplatin-induced caspase-3 activation.Toxicol In Vitro. 2012 Apr;26(3):435-44. doi: 10.1016/j.tiv.2012.01.014. Epub 2012 Jan 17. Toxicol In Vitro. 2012. PMID: 22269388
-
Distinct mechanisms contribute to acquired cisplatin resistance of urothelial carcinoma cells.Oncotarget. 2016 Jul 5;7(27):41320-41335. doi: 10.18632/oncotarget.9321. Oncotarget. 2016. PMID: 27191498 Free PMC article.
Cited by
-
Cell death and DNA damage via ROS mechanisms after applied antibiotics and antioxidants doses in prostate hyperplasia primary cell cultures.Medicine (Baltimore). 2024 Sep 13;103(37):e39450. doi: 10.1097/MD.0000000000039450. Medicine (Baltimore). 2024. PMID: 39287312 Free PMC article.
-
Imperatorin acts as a cisplatin sensitizer via downregulating Mcl-1 expression in HCC chemotherapy.Tumour Biol. 2016 Jan;37(1):331-9. doi: 10.1007/s13277-015-3591-z. Epub 2015 Jul 29. Tumour Biol. 2016. PMID: 26219890
-
Three-Dimensional Printed Cell Culture Model Based on Spherical Colloidal Lignin Particles and Cellulose Nanofibril-Alginate Hydrogel.Biomacromolecules. 2020 May 11;21(5):1875-1885. doi: 10.1021/acs.biomac.9b01745. Epub 2020 Feb 11. Biomacromolecules. 2020. PMID: 31992046 Free PMC article.
-
Differential effects of ginkgol C17:1 on cisplatin-induced cytotoxicity: Protecting human normal L02 hepatocytes versus sensitizing human hepatoma HepG2 cells.Oncol Lett. 2019 Mar;17(3):3181-3190. doi: 10.3892/ol.2019.9974. Epub 2019 Jan 25. Oncol Lett. 2019. PMID: 30867748 Free PMC article.
-
Human Organotypic Lung Tumor Models: Suitable For Preclinical 18F-FDG PET-Imaging.PLoS One. 2016 Aug 8;11(8):e0160282. doi: 10.1371/journal.pone.0160282. eCollection 2016. PLoS One. 2016. PMID: 27501455 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

