Effects of TGF-β1 on OPG/RANKL expression of cementoblasts and osteoblasts are similar without stress but different with mechanical compressive stress
- PMID: 25685846
- PMCID: PMC4312653
- DOI: 10.1155/2015/718180
Effects of TGF-β1 on OPG/RANKL expression of cementoblasts and osteoblasts are similar without stress but different with mechanical compressive stress
Abstract
Introduction: This study aimed to explore the effects of TGF-β1 on regulating activities of cementoblasts and osteoblasts with or without stress.
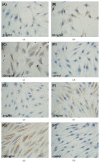
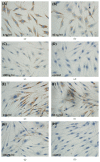
Material and methods: Human recombinant TGF-β1 was added with different doses. Immunohistochemical test of osteoprotegerin (OPG)/receptor activator of nuclear factor-kappaB ligand (RANKL) and Alizarin Red-S staining were conducted. Mechanical compressive stress was obtained by increasing the pressure of gaseous phase. OPG/RANKL expression was detected in both cells through quantitative real-time PCR.
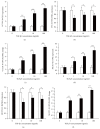
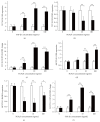
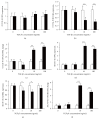
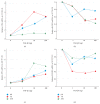
Results: Similar significant differences (P < 0.05) existed in OPG/RANKL change with increasing concentration of TGF-β1 without mechanical stress for cementoblasts and osteoblasts. However, under 3 h stress, OPG increased and RANKL decreased significantly (P < 0.01) but with similar OPG/RANKL change. Moreover, under 24 h stress, OPG change exhibited no difference (P > 0.05), but RANKL decreased significantly (P < 0.01) at 10 and 100 ng/mL TGF-β1 in cementoblasts. In osteoblasts, OPG increased significantly (P < 0.01) at 10 and 100 ng/mL, whereas RANKL decreased with statistical difference (P < 0.05) at 1 and 10 ng/mL.
Conclusions: The effects of TGF-β1 on OPG/RANKL expression of cementoblasts and osteoblasts are similar even without mechanical stress. However, these effects are different under mechanical compressive stress.
Figures


Similar articles
-
Effect of IL-1beta, PGE(2), and TGF-beta1 on the expression of OPG and RANKL in normal and osteoporotic primary human osteoblasts.J Cell Biochem. 2010 May 15;110(2):304-10. doi: 10.1002/jcb.22538. J Cell Biochem. 2010. PMID: 20225238
-
IL-1β and compressive forces lead to a significant induction of RANKL-expression in primary human cementoblasts.J Orofac Orthop. 2012 Sep;73(5):397-412. doi: 10.1007/s00056-012-0095-y. Epub 2012 Sep 7. J Orofac Orthop. 2012. PMID: 22955577
-
Effect of compressive loading and incubation with clodronate on the RANKL/OPG system of human osteoblasts.J Orofac Orthop. 2015 Nov;76(6):531-42. doi: 10.1007/s00056-015-0316-2. J Orofac Orthop. 2015. PMID: 26446504
-
Gu Ling Pian, a traditional Chinese medicine, regulates function and OPG/RANKL synthesis of osteoblasts via the p38 MAPK pathway.J Pharm Pharmacol. 2007 Aug;59(8):1167-73. doi: 10.1211/jpp.59.8.0016. J Pharm Pharmacol. 2007. PMID: 17725861
-
Prostate-specific antigen stimulates osteoprotegerin production and inhibits receptor activator of nuclear factor-kappaB ligand expression by human osteoblasts.Prostate. 2007 Jun 1;67(8):840-8. doi: 10.1002/pros.20574. Prostate. 2007. PMID: 17394194
Cited by
-
Platelet-rich fibrin increases the osteoprotegerin/receptor activator of nuclear factor-κB ligand ratio in osteoblasts.Exp Ther Med. 2019 Jul;18(1):358-365. doi: 10.3892/etm.2019.7560. Epub 2019 May 8. Exp Ther Med. 2019. PMID: 31258673 Free PMC article.
-
Shengu granules ameliorate ovariectomy-induced osteoporosis by the gut-bone-immune axis.Front Microbiol. 2024 Mar 8;15:1320500. doi: 10.3389/fmicb.2024.1320500. eCollection 2024. Front Microbiol. 2024. PMID: 38525084 Free PMC article.
-
Long noncoding RNA expression profile of mouse cementoblasts under compressive force.Angle Orthod. 2019 May;89(3):455-463. doi: 10.2319/061118-438.1. Epub 2019 Jan 2. Angle Orthod. 2019. PMID: 30605018 Free PMC article.
-
Expression and function of osteogenic genes runt-related transcription factor 2 and osterix in orthodontic tooth movement in rats.Int J Clin Exp Pathol. 2015 Sep 1;8(9):11895-900. eCollection 2015. Int J Clin Exp Pathol. 2015. PMID: 26617945 Free PMC article.
-
Modulating OPG and TGF-β1 mRNA expression via bioelectrical stimulation.Bone Rep. 2021 Oct 9;15:101141. doi: 10.1016/j.bonr.2021.101141. eCollection 2021 Dec. Bone Rep. 2021. PMID: 34692946 Free PMC article.
References
-
- Wang D., Christensen K., Chawla K., Xiao G., Krebsbach P. H., Franceschi R. T. Isolation and characterization of MC3T3-E1 preosteoblast subclones with distinct in vitro and in vivo differentiation/mineralization potential. Journal of Bone and Mineral Research. 1999;14(6):893–903. doi: 10.1359/jbmr.1999.14.6.893. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

