Melampodium leucanthum, a source of cytotoxic sesquiterpenes with antimitotic activities
- PMID: 25685941
- PMCID: PMC4749154
- DOI: 10.1021/np500768s
Melampodium leucanthum, a source of cytotoxic sesquiterpenes with antimitotic activities
Abstract
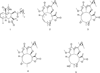
A new tricyclic sesquiterpene, named meleucanthin (1), was isolated from an extract of the leaves and branches of Melampodium leucanthum, along with four known germacranolide sesquiterpene lactones, leucanthin-A (2), leucanthin-B (3), melampodin-A acetate (4), and 3α-hydroxyenhydrin (5). The chemical structure of 1 was elucidated by analysis of 1D and 2D NMR and mass spectrometric data. All compounds exhibited antiproliferative and cytotoxic efficacy against PC-3 and DU 145 prostate cancer cells, as well as HeLa cervical cancer cells, with IC50 values ranging from 0.18 to 9 μM. These compounds were effective in clonogenic assays and displayed high cellular persistence. They were also found to be capable of circumventing P-glycoprotein-mediated drug resistance. Mechanism of action studies showed that 4 caused an accumulation of cells in the G2/M phase of the cell cycle, and 2-5 caused the formation of abnormal mitotic spindles. These results suggest the cytotoxic effects of these germacranolides involve inhibition of mitotic spindle function, and it is likely that other mechanisms additionally contribute to cell death. These studies also demonstrate the possibility of isolating new, biologically active compounds from indigenous Texas plants.
Figures







References
-
- Siegel R, Ma J, Zou Z, Jemal A. CA Cancer J. Clin. 2014;64:9–29. - PubMed
-
- Kirby M, Hirst C, Crawford ED. Int. J. Clin. Pract. 2011;65:1180–1192. - PubMed
-
- Cookson MS, Roth BJ, Dahm P, Engstrom C, Freedland SJ, Hussain M, Lin DW, Lowrance WT, Murad MH, Oh WK, Penson DF, Kibel AS. J. Urol. 2013;190:429–438. - PubMed
-
- Hwang SS, Chang VT, Alejandro Y, Mulaparthi S, Cogswell J, Srinivas S, Kasimis B. Cancer. Invest. 2004;22:849–857. - PubMed
-
- Soerdjbalie-Maikoe V, Pelger RC, Lycklama a Nijeholt GA, Arndt JW, Zwinderman AH, Bril H, Papapoulos SE, Hamdy NA. Eur. J. Nucl. Med. Mol. Imaging. 2004;31:958–963. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

