Transplanted Umbilical Cord Mesenchymal Stem Cells Modify the In Vivo Microenvironment Enhancing Angiogenesis and Leading to Bone Regeneration
- PMID: 25685989
- PMCID: PMC4499786
- DOI: 10.1089/scd.2014.0490
Transplanted Umbilical Cord Mesenchymal Stem Cells Modify the In Vivo Microenvironment Enhancing Angiogenesis and Leading to Bone Regeneration
Abstract
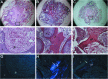
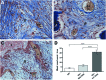
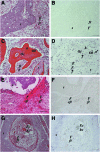
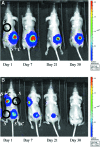
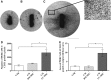
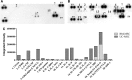
Umbilical cord mesenchymal stem cells (UC-MSCs) show properties similar to bone marrow mesenchymal stem cells (BM-MSCs), although controversial data exist regarding their osteogenic potential. We prepared clinical-grade UC-MSCs from Wharton's Jelly and we investigated if UC-MSCs could be used as substitutes for BM-MSCs in muscoloskeletal regeneration as a more readily available and functional source of MSCs. UC-MSCs were loaded onto scaffolds and implanted subcutaneously (ectopically) and in critical-sized calvarial defects (orthotopically) in mice. For live cell-tracking experiments, UC-MSCs were first transduced with the luciferase gene. Angiogenic properties of UC-MSCs were tested using the mouse metatarsal angiogenesis assay. Cell secretomes were screened for the presence of various cytokines using an array assay. Analysis of implanted scaffolds showed that UC-MSCs, contrary to BM-MSCs, remained detectable in the implants for 3 weeks at most and did not induce bone formation in an ectopic location. Instead, they induced a significant increase of blood vessel ingrowth. In agreement with these observations, UC-MSC-conditioned medium presented a distinct and stronger proinflammatory/chemotactic cytokine profile than BM-MSCs and a significantly enhanced angiogenic activity. When UC-MSCs were orthotopically transplanted in a calvarial defect, they promoted increased bone formation as well as BM-MSCs. However, at variance with BM-MSCs, the new bone was deposited through the activity of stimulated host cells, highlighting the importance of the microenvironment on determining cell commitment and response. Therefore, we propose, as therapy for bone lesions, the use of allogeneic UC-MSCs by not depositing bone matrix directly, but acting through the activation of endogenous repair mechanisms.
Figures

Similar articles
-
Functional module analysis reveals differential osteogenic and stemness potentials in human mesenchymal stem cells from bone marrow and Wharton's jelly of umbilical cord.Stem Cells Dev. 2010 Dec;19(12):1895-910. doi: 10.1089/scd.2009.0485. Epub 2010 Oct 12. Stem Cells Dev. 2010. PMID: 20367285
-
Endothelial differentiation of Wharton's jelly-derived mesenchymal stem cells in comparison with bone marrow-derived mesenchymal stem cells.Exp Hematol. 2009 May;37(5):629-40. doi: 10.1016/j.exphem.2009.02.003. Exp Hematol. 2009. PMID: 19375653
-
Role of VEGF-A in angiogenesis promoted by umbilical cord-derived mesenchymal stromal/stem cells: in vitro study.Stem Cell Res Ther. 2016 Mar 22;7:46. doi: 10.1186/s13287-016-0305-4. Stem Cell Res Ther. 2016. PMID: 27001300 Free PMC article.
-
Bone and cartilage regeneration with the use of umbilical cord mesenchymal stem cells.Expert Opin Biol Ther. 2015;15(11):1541-52. doi: 10.1517/14712598.2015.1068755. Epub 2015 Jul 15. Expert Opin Biol Ther. 2015. PMID: 26176327 Review.
-
The effects of microenvironment in mesenchymal stem cell-based regeneration of intervertebral disc.Spine J. 2013 Mar;13(3):352-62. doi: 10.1016/j.spinee.2012.12.005. Epub 2013 Jan 20. Spine J. 2013. PMID: 23340343 Review.
Cited by
-
Quantification of the CM-Dil-labeled human umbilical cord mesenchymal stem cells migrated to the dual injured uterus in SD rat.Stem Cell Res Ther. 2020 Jul 13;11(1):280. doi: 10.1186/s13287-020-01806-4. Stem Cell Res Ther. 2020. PMID: 32660551 Free PMC article.
-
Immunomodulatory oligonucleotide IMT504: Effects on mesenchymal stem cells as a first-in-class immunoprotective/immunoregenerative therapy.World J Stem Cells. 2017 Mar 26;9(3):45-67. doi: 10.4252/wjsc.v9.i3.45. World J Stem Cells. 2017. PMID: 28396715 Free PMC article. Review.
-
IL-1b in the Secretomes of MSCs Seeded on Human Decellularized Allogeneic Bone Promotes Angiogenesis.Int J Mol Sci. 2022 Dec 4;23(23):15301. doi: 10.3390/ijms232315301. Int J Mol Sci. 2022. PMID: 36499629 Free PMC article.
-
Human umbilical cord mesenchymal stromal cells-derived extracellular vesicles exert potent bone protective effects by CLEC11A-mediated regulation of bone metabolism.Theranostics. 2020 Jan 16;10(5):2293-2308. doi: 10.7150/thno.39238. eCollection 2020. Theranostics. 2020. PMID: 32089743 Free PMC article.
-
Regional Gene Therapy for Bone Tissue Engineering: A Current Concepts Review.Bioengineering (Basel). 2025 Jan 27;12(2):120. doi: 10.3390/bioengineering12020120. Bioengineering (Basel). 2025. PMID: 40001640 Free PMC article. Review.
References
-
- Mauney JR, Ph D, Volloch V. and Kaplan DL. (2005). Role of adult mesenchymal stem cells in bone tissue-engineering applications: current status and future prospects. Tissue Eng 11:787–802 - PubMed
-
- El Backly RM, Zaky SH, Muraglia A, Tonachini L, Brun F, Canciani B, Chiapale D, Santolini F, Cancedda R. and Mastrogiacomo M. (2013). A platelet-rich plasma-based membrane as a periosteal substitute with enhanced osteogenic and angiogenic properties: a new concept for bone repair. Tissue Eng Part A 19:152–165 - PubMed
-
- Giannoni P, Mastrogiacomo M, Alini M, Pearce SG, Corsi A, Santolini F, Muraglia A, Bianco P. and Cancedda R. (2008). Regeneration of large bone defects in sheep using bone marrow stromal cells. J Tissue Eng Regen Med 2:253–262 - PubMed
-
- Quarto R, Mastrogiacomo M, Cancedda R, Kutepov SM, Mukhachev V, Lavroukov A, Kon E. and Marcacci M. (2001). Repair of large bone defects with the use of autologous bone marrow stromal cells. N Engl J Med 344:385–386 - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

