Structural basis for bifunctional peptide recognition at human δ-opioid receptor
- PMID: 25686086
- PMCID: PMC4351130
- DOI: 10.1038/nsmb.2965
Structural basis for bifunctional peptide recognition at human δ-opioid receptor
Abstract
Bifunctional μ- and δ-opioid receptor (OR) ligands are potential therapeutic alternatives, with diminished side effects, to alkaloid opiate analgesics. We solved the structure of human δ-OR bound to the bifunctional δ-OR antagonist and μ-OR agonist tetrapeptide H-Dmt-Tic-Phe-Phe-NH2 (DIPP-NH2) by serial femtosecond crystallography, revealing a cis-peptide bond between H-Dmt and Tic. The observed receptor-peptide interactions are critical for understanding of the pharmacological profiles of opioid peptides and for development of improved analgesics.
Figures
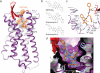
Comment in
-
Serial femtosecond crystallography datasets from G protein-coupled receptors.Sci Data. 2016 Aug 1;3:160057. doi: 10.1038/sdata.2016.57. Sci Data. 2016. PMID: 27479354 Free PMC article.
References
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
- Y1-GM-1104/GM/NIGMS NIH HHS/United States
- U54 GM094599/GM/NIGMS NIH HHS/United States
- R01 GM095583/GM/NIGMS NIH HHS/United States
- R01 DA017204/DA/NIDA NIH HHS/United States
- U54 GM094618/GM/NIGMS NIH HHS/United States
- Y1-CO-1020/CO/NCI NIH HHS/United States
- R01 GM108635/GM/NIGMS NIH HHS/United States
- R37 DA004443/DA/NIDA NIH HHS/United States
- P01 DA035764/DA/NIDA NIH HHS/United States
- P41 GM103393/GM/NIGMS NIH HHS/United States
- R21 DA038858/DA/NIDA NIH HHS/United States
- R01 DA004443/DA/NIDA NIH HHS/United States
- U19 MH082441/MH/NIMH NIH HHS/United States
- R33 DA038858/DA/NIDA NIH HHS/United States
- MOP-89716/CAPMC/ CIHR/Canada
- DA-004443/DA/NIDA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

