Application of GFP imaging in cancer
- PMID: 25686095
- PMCID: PMC4383682
- DOI: 10.1038/labinvest.2014.154
Application of GFP imaging in cancer
Abstract
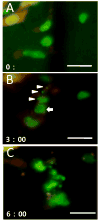
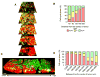
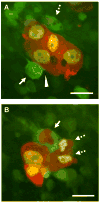
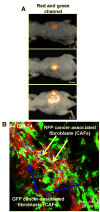
Multicolored proteins have allowed the color-coding of cancer cells growing in vivo and enabled the distinction of host from tumor with single-cell resolution. Non-invasive imaging with fluorescent proteins enabled the dynamics of metastatic cancer to be followed in real time in individual animals. Non-invasive imaging of cancer cells expressing fluorescent proteins has allowed the real-time determination of efficacy of candidate antitumor and antimetastatic agents in mouse models. The use of fluorescent proteins to differentially label cancer cells in the nucleus and cytoplasm can visualize the nuclear-cytoplasmic dynamics of cancer cells in vivo including: mitosis, apoptosis, cell-cycle position, and differential behavior of nucleus and cytoplasm that occurs during cancer-cell deformation and extravasation. Recent applications of the technology described here include linking fluorescent proteins with cell-cycle-specific proteins such that the cells change color from red to green as they transit from G1 to S phases. With the macro- and micro-imaging technologies described here, essentially any in vivo process can be imaged, giving rise to the new field of in vivo cell biology using fluorescent proteins.
Conflict of interest statement
Figures










Similar articles
-
Fluorescent Proteins as Sensors for Cellular Behavior in Mice.Prog Mol Biol Transl Sci. 2018;160:29-45. doi: 10.1016/bs.pmbts.2018.09.005. Epub 2018 Nov 12. Prog Mol Biol Transl Sci. 2018. PMID: 30470291 Review.
-
Fluorescent proteins as visible in vivo sensors.Prog Mol Biol Transl Sci. 2013;113:389-402. doi: 10.1016/B978-0-12-386932-6.00010-7. Prog Mol Biol Transl Sci. 2013. PMID: 23244796 Review.
-
Tumor imaging with multicolor fluorescent protein expression.Int J Clin Oncol. 2011 Apr;16(2):84-91. doi: 10.1007/s10147-011-0201-y. Epub 2011 Feb 25. Int J Clin Oncol. 2011. PMID: 21347627 Review.
-
The Use of Living Cancer Cells Expressing Green Fluorescent Protein in the Nucleus and Red Fluorescence Protein in the Cytoplasm for Real-time Confocal Imaging of Chromosome and Cytoplasmic Dynamics During Mitosis.Anticancer Res. 2015 May;35(5):2553-7. Anticancer Res. 2015. PMID: 25964529
-
Strategies for In Vivo Imaging Using Fluorescent Proteins.J Cell Biochem. 2017 Sep;118(9):2571-2580. doi: 10.1002/jcb.25677. Epub 2017 May 25. J Cell Biochem. 2017. PMID: 27564860 Review.
Cited by
-
A Comprehensive Exploration of Bioluminescence Systems, Mechanisms, and Advanced Assays for Versatile Applications.Biochem Res Int. 2024 Feb 5;2024:8273237. doi: 10.1155/2024/8273237. eCollection 2024. Biochem Res Int. 2024. PMID: 38347947 Free PMC article. Review.
-
Fluorescence Imaging as a Tool in Preclinical Evaluation of Polymer-Based Nano-DDS Systems Intended for Cancer Treatment.Pharmaceutics. 2019 Sep 12;11(9):471. doi: 10.3390/pharmaceutics11090471. Pharmaceutics. 2019. PMID: 31547308 Free PMC article. Review.
-
Anti-Prostate Cancer Activity of 8-Hydroxyquinoline-2-Carboxaldehyde-Thiosemicarbazide Copper Complexes by Fluorescent Microscopic Imaging.J Fluoresc. 2017 Nov;27(6):1937-1941. doi: 10.1007/s10895-017-2133-z. Epub 2017 Jun 26. J Fluoresc. 2017. PMID: 28653241
-
Near Infrared Photoimmunotherapy with Combined Exposure of External and Interstitial Light Sources.Mol Pharm. 2018 Sep 4;15(9):3634-3641. doi: 10.1021/acs.molpharmaceut.8b00002. Epub 2018 Feb 21. Mol Pharm. 2018. PMID: 29450993 Free PMC article.
-
Deep tissue imaging: a review from a preclinical cancer research perspective.Histochem Cell Biol. 2016 Dec;146(6):781-806. doi: 10.1007/s00418-016-1495-7. Epub 2016 Oct 4. Histochem Cell Biol. 2016. PMID: 27704211 Review.
References
-
- Hoffman RM. The multiple uses of fluorescent proteins to visualize cancer in vivo. Nature Reviews Cancer. 2005;5:796–806. - PubMed
-
- Shcherbo D, Merzlyak EM, Chepurnykh TV, et al. Bright far-red fluorescent protein for whole-body imaging. Nat Methods. 2007;4:741–746. - PubMed
-
- Yamamoto N, Yang M, Jiang P, et al. Real-time imaging of individual color-coded metastatic colonies in vivo. Clin Exp Metastasis. 2003;20:633–638. - PubMed
-
- Brown EB, Campbell RB, Tsuzuki Y, et al. In vivo measurement of gene expression, angiogenesis and physiological function in tumors using multiphoton laser scanning microscopy. Nature Med. 2001;7:864–868. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

