Establishing the Lysine-rich Protein CEST Reporter Gene as a CEST MR Imaging Detector for Oncolytic Virotherapy
- PMID: 25686366
- PMCID: PMC4450912
- DOI: 10.1148/radiol.14140251
Establishing the Lysine-rich Protein CEST Reporter Gene as a CEST MR Imaging Detector for Oncolytic Virotherapy
Abstract
Purpose: To (a) evaluate whether the lysine-rich protein (LRP) magnetic resonance (MR) imaging reporter gene can be engineered into G47Δ, a herpes simplex-derived oncolytic virus that is currently being tested in clinical trials, without disrupting its therapeutic effectiveness and (b) establish the ability of chemical exchange saturation transfer (CEST) MR imaging to demonstrate G47Δ-LRP.
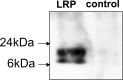
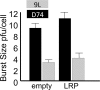
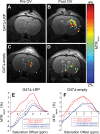
Materials and methods: The institutional subcommittee for research animal care approved all in vivo procedures. Oncolytic herpes simplex virus G47Δ, which carried the LRP gene, was constructed and tested for its capacity to replicate in cancer cells and express LRP in vitro. The LRP gene was detected through CEST imaging of lysates derived from cells infected with G47Δ-LRP or the control G47Δ-empty virus. G47Δ-LRP was then tested for its therapeutic effectiveness and detection with CEST MR imaging in vivo. Images of rat gliomas were acquired before and 8-10 hours after injection of G47Δ-LRP (n = 7) or G47Δ-empty virus (n = 6). Group comparisons were analyzed with a paired t test.
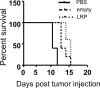
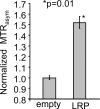
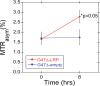
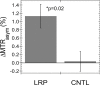
Results: No significant differences were observed in viral replication or therapeutic effectiveness between G47Δ-LRP and G47Δ-empty virus. An increase in CEST image contrast was observed in cell lysates (mean ± standard deviation, 0.52% ± 0.06; P = .01) and in tumors (1.1% ± 0.3, P = .02) after infection with G47Δ-LRP but not G47Δ-empty viruses. No histopathologic differences were observed between tumors infected with G47Δ-LRP and G47Δ-empty virus.
Conclusion: This study has demonstrated the ability of CEST MR imaging to show G47Δ-LRP at acute stages of viral infection. The introduction of the LRP transgene had no effect on the viral replication or therapeutic effectiveness. This can aid in development of the LRP gene as a reporter for the real-time detection of viral spread. Online supplemental material is available for this article.
RSNA, 2015
Figures



Comment in
-
Science to Practice: Monitoring Oncolytic Virus Therapy with Chemical Exchange Saturation Transfer MR Imaging--Wishful Thinking?Radiology. 2015 Jun;275(3):625-6. doi: 10.1148/radiol.2015150537. Radiology. 2015. PMID: 25997128 Free PMC article.
References
-
- Gilad AA, McMahon MT, Walczak P, et al. Artificial reporter gene providing MRI contrast based on proton exchange. Nat Biotechnol 2007;25(2):217–219. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

