Trapping of intermediates with substrate analog HBOCoA in the polymerizations catalyzed by class III polyhydroxybutyrate (PHB) synthase from Allochromatium vinosum
- PMID: 25686368
- PMCID: PMC4870837
- DOI: 10.1021/cb5009958
Trapping of intermediates with substrate analog HBOCoA in the polymerizations catalyzed by class III polyhydroxybutyrate (PHB) synthase from Allochromatium vinosum
Abstract
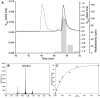
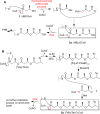
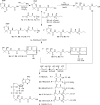
Polyhydroxybutyrate (PHB) synthases (PhaCs) catalyze the formation of biodegradable PHB polymers that are considered as an ideal alternative to petroleum-based plastics. To provide strong evidence for the preferred mechanistic model involving covalent and noncovalent intermediates, a substrate analog HBOCoA was synthesized chemoenzymatically. Substitution of sulfur in the native substrate HBCoA with an oxygen in HBOCoA enabled detection of (HB)nOCoA (n = 2-6) intermediates when the polymerization was catalyzed by wild-type (wt-)PhaECAv at 5.84 h(-1). This extremely slow rate is due to thermodynamically unfavorable steps that involve the formation of enzyme-bound PHB species (thioesters) from corresponding CoA oxoesters. Synthesized standards (HB)nOCoA (n = 2-3) were found to undergo both reacylation and hydrolysis catalyzed by the synthase. Distribution of the hydrolysis products highlights the importance of the penultimate ester group as previously suggested. Importantly, the reaction between primed synthase [(3)H]-sT-PhaECAv and HBOCoA yielded [(3)H]-sTet-O-CoA at a rate constant faster than 17.4 s(-1), which represents the first example that a substrate analog undergoes PHB chain elongation at a rate close to that of the native substrate (65.0 s(-1)). Therefore, for the first time with a wt-synthase, strong evidence was obtained to support our favored PHB chain elongation model.
Figures




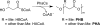

References
-
- Chee JY, Yoga SS, Lau NS, Ling SC, Abed RMM, Sudesh K. Bacterially produced polyhydroxyalkanoate (PHA): Converting renewable resources into bioplastics. Vol. 1. Formatex Research Center; Spain: 2010.
-
- Chen GQ. Plastics from bacteria: Natural functions and applications. Springer; Heidelberg ; New York: 2010.
-
- Verlinden RAJ, Hill DJ, Kenward MA, Williams CD, Radecka I. Bacterial synthesis of biodegradable polyhydroxyalkanoates. J. Appl. Microbiol. 2007;102:1437–1449. - PubMed
-
- Sudesh K, Abe H, Doi Y. Synthesis, structure and properties of polyhydroxyalkanoates: biological polyesters. Prog. Polym. Sci. 2000;25:1503–1555.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

