Proton radiobiology
- PMID: 25686476
- PMCID: PMC4381263
- DOI: 10.3390/cancers7010353
Proton radiobiology
Abstract
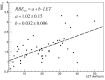
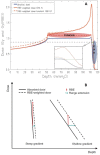
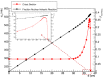
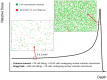
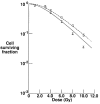
In addition to the physical advantages (Bragg peak), the use of charged particles in cancer therapy can be associated with distinct biological effects compared to X-rays. While heavy ions (densely ionizing radiation) are known to have an energy- and charge-dependent increased Relative Biological Effectiveness (RBE), protons should not be very different from sparsely ionizing photons. A slightly increased biological effectiveness is taken into account in proton treatment planning by assuming a fixed RBE of 1.1 for the whole radiation field. However, data emerging from recent studies suggest that, for several end points of clinical relevance, the biological response is differentially modulated by protons compared to photons. In parallel, research in the field of medical physics highlighted how variations in RBE that are currently neglected might actually result in deposition of significant doses in healthy organs. This seems to be relevant in particular for normal tissues in the entrance region and for organs at risk close behind the tumor. All these aspects will be considered and discussed in this review, highlighting how a re-discussion of the role of a variable RBE in proton therapy might be well-timed.
Figures

References
-
- Jermann M. Particle therapy statistics in 2013. Int. J. Part. Ther. 2014;1:40–43. doi: 10.14338/IJPT.14-editorial-2.1. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

