Static stretch affects neural stem cell differentiation in an extracellular matrix-dependent manner
- PMID: 25686615
- PMCID: PMC4330529
- DOI: 10.1038/srep08499
Static stretch affects neural stem cell differentiation in an extracellular matrix-dependent manner
Abstract
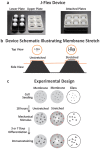
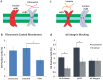
Neural stem and progenitor cell (NSPC) fate is strongly influenced by mechanotransduction as modulation of substrate stiffness affects lineage choice. Other types of mechanical stimuli, such as stretch (tensile strain), occur during CNS development and trauma, but their consequences for NSPC differentiation have not been reported. We delivered a 10% static equibiaxial stretch to NSPCs and examined effects on differentiation. We found static stretch specifically impacts NSPC differentiation into oligodendrocytes, but not neurons or astrocytes, and this effect is dependent on particular extracellular matrix (ECM)-integrin linkages. Generation of oligodendrocytes from NSPCs was reduced on laminin, an outcome likely mediated by the α6 laminin-binding integrin, whereas similar effects were not observed for NSPCs on fibronectin. Our data demonstrate a direct role for tensile strain in dictating the lineage choice of NSPCs and indicate the dependence of this phenomenon on specific substrate materials, which should be taken into account for the design of biomaterials for NSPC transplantation.
Figures



References
-
- Gage F. H. Mammalian neural stem cells. Science 287, 1433–1438 (2000). - PubMed
-
- Engler A., Sen S., Sweeney H. & Discher D. Matrix elasticity directs stem cell lineage specification. Cell 126, 677–689 (2006). - PubMed
-
- Tyler W. J. The mechanobiology of brain function. Nat Rev Neurosci 13, 867–878 (2012). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

