Epoxide hydrolase activities and epoxy fatty acids in the mosquito Culex quinquefasciatus
- PMID: 25686802
- PMCID: PMC4387068
- DOI: 10.1016/j.ibmb.2015.02.004
Epoxide hydrolase activities and epoxy fatty acids in the mosquito Culex quinquefasciatus
Abstract
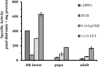
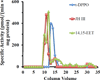
Culex mosquitoes have emerged as important model organisms for mosquito biology, and are disease vectors for multiple mosquito-borne pathogens, including West Nile virus. We characterized epoxide hydrolase activities in the mosquito Culex quinquefasciatus, which suggested multiple forms of epoxide hydrolases were present. We found EH activities on epoxy eicosatrienoic acids (EETs). EETs and other eicosanoids are well-established lipid signaling molecules in vertebrates. We showed EETs can be synthesized in vitro from arachidonic acids by mosquito lysate, and EETs were also detected in vivo both in larvae and adult mosquitoes by LC-MS/MS. The EH activities on EETs can be induced by blood feeding, and the highest activity was observed in the midgut of female mosquitoes. The enzyme activities on EETs can be inhibited by urea-based inhibitors designed for mammalian soluble epoxide hydrolases (sEH). The sEH inhibitors have been shown to play diverse biological roles in mammalian systems, and they can be useful tools to study the function of EETs in mosquitoes. Besides juvenile hormone metabolism and detoxification, insect epoxide hydrolases may also play a role in regulating lipid signaling molecules, such as EETs and other epoxy fatty acids, synthesized in vivo or obtained from blood feeding by female mosquitoes.
Keywords: Eicosanoid; Epoxide hydrolase; Epoxy fatty acid; Inhibitor; Mosquito; Signaling molecule.
Copyright © 2015 Elsevier Ltd. All rights reserved.
Figures







References
-
- Abdel-Aal YAI, Hammock BD. 3-octylthio-1,1,1-trifluoro- 2-propanone, a high affinity and slow binding inhibitor of juvenile hormone esterase from Trichoplusia ni (hübner) Insect Biochemistry. 1985;15:111–122.
-
- Anspaugh DD, Roe RM. Regulation of JH epoxide hydrolase versus JH esterase activity in the cabbage looper, Trichoplusia ni, by juvenile hormone and xenobiotics. Journal of Insect Physiology. 2005;51:523–535. - PubMed
-
- Arand M, Wagner H, Oesch F. Asp333, Asp495, and His523 form the catalytic triad of rat soluble epoxide hydrolase. The Journal of biological chemistry. 1996;271:4223–4229. - PubMed
-
- Arensburger P, Megy K, Waterhouse RM, Abrudan J, Amedeo P, Antelo B, Bartholomay L, Bidwell S, Caler E, Camara F, Campbell CL, Campbell KS, Casola C, Castro MT, Chandramouliswaran I, Chapman SB, Christley S, Costas J, Eisenstadt E, Feschotte C, Fraser-Liggett C, Guigo R, Haas B, Hammond M, Hansson BS, Hemingway J, Hill SR, Howarth C, Ignell R, Kennedy RC, Kodira CD, Lobo NF, Mao C, Mayhew G, Michel K, Mori A, Liu N, Naveira H, Nene V, Nguyen N, Pearson MD, Pritham EJ, Puiu D, Qi Y, Ranson H, Ribeiro JM, Roberston HM, Severson DW, Shumway M, Stanke M, Strausberg RL, Sun C, Sutton G, Tu ZJ, Tubio JM, Unger MF, Vanlandingham DL, Vilella AJ, White O, White JR, Wondji CS, Wortman J, Zdobnov EM, Birren B, Christensen BM, Collins FH, Cornel A, Dimopoulos G, Hannick LI, Higgs S, Lanzaro GC, Lawson D, Lee NH, Muskavitch MA, Raikhel AS, Atkinson PW. Sequencing of Culex quinquefasciatus establishes a platform for mosquito comparative genomics. Science. 2010;330:86–88. - PMC - PubMed
-
- Bartholomay LC, Waterhouse RM, Mayhew GF, Campbell CL, Michel K, Zou Z, Ramirez JL, Das S, Alvarez K, Arensburger P, Bryant B, Chapman SB, Dong Y, Erickson SM, Karunaratne SH, Kokoza V, Kodira CD, Pignatelli P, Shin SW, Vanlandingham DL, Atkinson PW, Birren B, Christophides GK, Clem RJ, Hemingway J, Higgs S, Megy K, Ranson H, Zdobnov EM, Raikhel AS, Christensen BM, Dimopoulos G, Muskavitch MA. Pathogenomics of Culex quinquefasciatus and meta-analysis of infection responses to diverse pathogens. Science. 2010;330:88–90. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

