Initial in vivo experience of pig artery patch transplantation in baboons using mutant MHC (CIITA-DN) pigs
- PMID: 25687023
- PMCID: PMC4368496
- DOI: 10.1016/j.trim.2015.02.003
Initial in vivo experience of pig artery patch transplantation in baboons using mutant MHC (CIITA-DN) pigs
Abstract
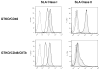
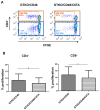
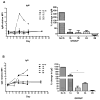
Background: In the pig-to-nonimmunosuppressed baboon artery patch model, a graft from an α1,3-galactosyltransferase gene-knockout pig transgenic for human CD46 (GTKO/CD46) induces a significant adaptive immune response (elicited anti-pig antibody response, increase in T cell proliferation on MLR, cellular infiltration of the graft), which is effectively prevented by anti-CD154mAb-based therapy.
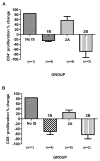
Methods: As anti-CD154mAb is currently not clinically applicable, we evaluated whether it could be replaced by CD28/B7 pathway blockade or by blockade of both pathways (using belatacept + anti-CD40mAb [2C10R4]). We further investigated whether a patch from a GTKO/CD46 pig with a mutant human MHC class II transactivator (CIITA-DN) gene would allow reduction in the immunosuppressive therapy administered.
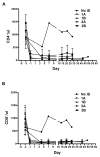
Results: When grafts from GTKO/CD46 pigs were transplanted with blockade of both pathways, a minimal or insignificant adaptive response was documented. When a GTKO/CD46/CIITA-DN graft was transplanted, but no immunosuppressive therapy was administered, a marked adaptive response was documented. In the presence of CD28/B7 pathway blockade (abatacept or belatacept), there was a weak adaptive response that was diminished when compared with that to a GTKO/CD46 graft. Blockade of both pathways prevented an adaptive response.
Conclusion: Although expression of the mutant MHC CIITA-DN gene was associated with a reduced adaptive immune response when immunosuppressive therapy was inadequate, when blockade of both the CD40/CD154 and CD28/B7 pathways was present, the response even to a GTKO/CD46 graft was suppressed. This was confirmed after GTKO/CD46 heart transplantation in baboons.
Keywords: Anti-CD40 monoclonal antibody; Artery patch; CTLA4-Ig; Costimulation blockade; Pig; Xenotransplantation.
Copyright © 2015 Elsevier B.V. All rights reserved.
Conflict of interest statement
Carol Phelps and David Ayares are employees of Revivicor. No other author has a conflict of interest.
Figures




Similar articles
-
B cell phenotypes in baboons with pig artery patch grafts receiving conventional immunosuppressive therapy.Transpl Immunol. 2018 Dec;51:12-20. doi: 10.1016/j.trim.2018.08.005. Epub 2018 Aug 6. Transpl Immunol. 2018. PMID: 30092338 Free PMC article.
-
Costimulation blockade in pig artery patch xenotransplantation - a simple model to monitor the adaptive immune response in nonhuman primates.Xenotransplantation. 2012 Jul-Aug;19(4):221-32. doi: 10.1111/j.1399-3089.2012.00711.x. Xenotransplantation. 2012. PMID: 22909135 Free PMC article.
-
Pig-to-baboon heterotopic heart transplantation--exploratory preliminary experience with pigs transgenic for human thrombomodulin and comparison of three costimulation blockade-based regimens.Xenotransplantation. 2015 May-Jun;22(3):211-20. doi: 10.1111/xen.12167. Epub 2015 Apr 3. Xenotransplantation. 2015. PMID: 25847282 Free PMC article.
-
The pathobiology of pig-to-primate xenotransplantation: a historical review.Xenotransplantation. 2016 Mar;23(2):83-105. doi: 10.1111/xen.12219. Epub 2016 Jan 27. Xenotransplantation. 2016. PMID: 26813438 Review.
-
New concepts of immune modulation in xenotransplantation.Transplantation. 2013 Dec 15;96(11):937-45. doi: 10.1097/TP.0b013e31829bbcb2. Transplantation. 2013. PMID: 23851935 Free PMC article. Review.
Cited by
-
Experimental hepatocyte xenotransplantation--a comprehensive review of the literature.Xenotransplantation. 2015 Jul-Aug;22(4):239-48. doi: 10.1111/xen.12170. Epub 2015 May 7. Xenotransplantation. 2015. PMID: 25950141 Free PMC article. Review.
-
Thyroid hormone: relevance to xenotransplantation.Xenotransplantation. 2016 Jul;23(4):293-9. doi: 10.1111/xen.12243. Epub 2016 Jul 4. Xenotransplantation. 2016. PMID: 27374212 Free PMC article.
-
In Search of the Ideal Valve: Optimizing Genetic Modifications to Prevent Bioprosthetic Degeneration.Ann Thorac Surg. 2019 Aug;108(2):624-635. doi: 10.1016/j.athoracsur.2019.01.054. Epub 2019 Mar 2. Ann Thorac Surg. 2019. PMID: 30836101 Free PMC article. Review.
-
Initial in vitro studies on tissues and cells from GTKO/CD46/NeuGcKO pigs.Xenotransplantation. 2016 Mar;23(2):137-50. doi: 10.1111/xen.12229. Epub 2016 Mar 14. Xenotransplantation. 2016. PMID: 26988899 Free PMC article.
-
The role of genetically engineered pigs in xenotransplantation research.J Pathol. 2016 Jan;238(2):288-99. doi: 10.1002/path.4635. Epub 2015 Oct 7. J Pathol. 2016. PMID: 26365762 Free PMC article. Review.
References
-
- Cooper DK, Good AH, Koren E, Oriol R, Malcolm AJ, Ippolito RM, et al. Identification of alpha-galactosyl and other carbohydrate epitopes that are bound by human anti-pig antibodies: relevance to discordant xenografting in man. Transpl Immunol. 1993;1:198–205. - PubMed
-
- Cooper DK, Koren E, Oriol R. Genetically engineered pigs. Lancet. 1993;342:682–3. - PubMed
-
- Cozzi E, White DJ. The generation of transgenic pigs as potential organ donors for humans. Nat Med. 1995;1:964–6. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- # R21 A1074844/PHS HHS/United States
- UL1 TR000005/TR/NCATS NIH HHS/United States
- R21 AI074844/AI/NIAID NIH HHS/United States
- T32 AI074490/AI/NIAID NIH HHS/United States
- #U01 AI068642/AI/NIAID NIH HHS/United States
- P40 OD010988/OD/NIH HHS/United States
- U01 AI068642/AI/NIAID NIH HHS/United States
- P40 OD010431/OD/NIH HHS/United States
- P01 HL107152/HL/NHLBI NIH HHS/United States
- #U19 AI090959/AI/NIAID NIH HHS/United States
- P40OD010431/OD/NIH HHS/United States
- # PO1 HL107152/HL/NHLBI NIH HHS/United States
- P40OD010988/OD/NIH HHS/United States
- U19 AI090959/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

