Estrogen promotes luteolysis by redistributing prostaglandin F2α receptors within primate luteal cells
- PMID: 25687410
- PMCID: PMC4380810
- DOI: 10.1530/REP-14-0412
Estrogen promotes luteolysis by redistributing prostaglandin F2α receptors within primate luteal cells
Abstract
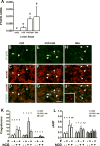
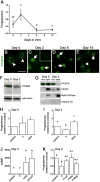
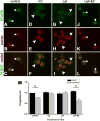
Prostaglandin F2α (PGF2α) has been proposed as a functional luteolysin in primates. However, administration of PGF2α or prostaglandin synthesis inhibitors in vivo both initiate luteolysis. These contradictory findings may reflect changes in PGF2α receptors (PTGFRs) or responsiveness to PGF2α at a critical point during the life span of the corpus luteum. The current study addressed this question using ovarian cells and tissues from female cynomolgus monkeys and luteinizing granulosa cells from healthy women undergoing follicle aspiration. PTGFRs were present in the cytoplasm of monkey granulosa cells, while PTGFRs were localized in the perinuclear region of large, granulosa-derived monkey luteal cells by mid-late luteal phase. A PTGFR agonist decreased progesterone production in luteal cells obtained at mid-late and late luteal phases, but did not decrease progesterone production by granulosa cells or luteal cells from younger corpora lutea. These findings are consistent with a role for perinuclear PTGFRs in functional luteolysis. This concept was explored using human luteinizing granulosa cells maintained in vitro as a model for luteal cell differentiation. In these cells, PTGFRs relocated from the cytoplasm to the perinuclear area in an estrogen- and estrogen receptor-dependent manner. Similar to our findings with monkey luteal cells, human luteinizing granulosa cells with perinuclear PTGFRs responded to a PTGFR agonist with decreased progesterone production. These data support the concept that PTGFR stimulation promotes functional luteolysis only when PTGFRs are located in the perinuclear region. Estrogen receptor-mediated relocation of PTGFRs within luteal cells may be a necessary step in the initiation of luteolysis in primates.
© 2015 Society for Reproduction and Fertility.
Conflict of interest statement
DISCLOSURE STATEMENT: The authors have nothing to disclose.
Figures

Similar articles
-
Expression of prostaglandin F2α (PGF2α) receptor and its isoforms in the bovine corpus luteum during the estrous cycle and PGF2α-induced luteolysis.Domest Anim Endocrinol. 2012 Oct;43(3):227-38. doi: 10.1016/j.domaniend.2012.03.003. Epub 2012 Apr 12. Domest Anim Endocrinol. 2012. PMID: 22560179
-
The impact of flutamide on prostaglandin F2α synthase and prostaglandin F2α receptor expression, and prostaglandin F2α concentration in the porcine corpus luteum of pregnancy.Domest Anim Endocrinol. 2017 Apr;59:81-89. doi: 10.1016/j.domaniend.2016.12.001. Epub 2016 Dec 11. Domest Anim Endocrinol. 2017. PMID: 28038404
-
Increased 27-hydroxycholesterol production during luteolysis may mediate the progressive decline in progesterone secretion.Mol Hum Reprod. 2018 Jan 1;24(1):2-13. doi: 10.1093/molehr/gax061. Mol Hum Reprod. 2018. PMID: 29177442 Free PMC article.
-
Judge, jury and executioner: the auto-regulation of luteal function.Soc Reprod Fertil Suppl. 2007;64:191-206. doi: 10.5661/rdr-vi-191. Soc Reprod Fertil Suppl. 2007. PMID: 17491148 Review.
-
Luteolysis: a neuroendocrine-mediated event.Physiol Rev. 1999 Apr;79(2):263-323. doi: 10.1152/physrev.1999.79.2.263. Physiol Rev. 1999. PMID: 10221982 Review.
Cited by
-
Prostaglandin F2α regulates mitochondrial dynamics and mitophagy in the bovine corpus luteum.Life Sci Alliance. 2023 May 15;6(7):e202301968. doi: 10.26508/lsa.202301968. Print 2023 Jul. Life Sci Alliance. 2023. PMID: 37188480 Free PMC article.
-
Ovulation: Parallels With Inflammatory Processes.Endocr Rev. 2019 Apr 1;40(2):369-416. doi: 10.1210/er.2018-00075. Endocr Rev. 2019. PMID: 30496379 Free PMC article. Review.
-
Luteotropic and Luteolytic Factors Modulate the Expression of Nuclear Receptor Coregulators in Bovine Luteal Cells Independently of Histone Acetyltransferase and Histone Deacetylase Activities.Animals (Basel). 2023 Aug 31;13(17):2784. doi: 10.3390/ani13172784. Animals (Basel). 2023. PMID: 37685048 Free PMC article.
-
Unraveling the role of lipid droplets and perilipin 2 in bovine luteal cells.FASEB J. 2024 Jun 15;38(11):e23710. doi: 10.1096/fj.202400260RR. FASEB J. 2024. PMID: 38822676 Free PMC article.
-
Thrombospondin 1 (THBS1) Promotes Follicular Angiogenesis, Luteinization, and Ovulation in Primates.Front Endocrinol (Lausanne). 2019 Nov 7;10:727. doi: 10.3389/fendo.2019.00727. eCollection 2019. Front Endocrinol (Lausanne). 2019. PMID: 31787928 Free PMC article.
References
-
- Abayasekara DRE, Michael AE, Webley GE, Flint APF. Mode of action of prostaglandin F2α in human luteinized granulosa cells: role of protein kinase C. Molecular and Cellular Endocrinology. 1993;97:81–91. - PubMed
-
- Arvisais EW, Romanelli A, Hou X, Davis JS. AKT-independent phosphorylation of TSC2 and activation of mTOR and ribosomal protein S6 kinase signaling by prostaglandin F2α. 2006;281:26904–26913. - PubMed
-
- Auletta FJ, Flint APF. Mechanisms controlling corpus luteum function in sheep, cows, nonhuman primates, and women especially in relation to the time of luteolysis. Endocrine Reviews. 1988;9:88–105. - PubMed
-
- Auletta FJ, Kelm LB, Schofield MJ. Responsiveness of the corpus luteum of the rhesus monkey (Macaca mulatta) to gonadotrophin in vitro during spontaneous and prostaglandin F2α-induced luteolysis. Journal of Reproduction and Fertility. 1995;103:107–113. - PubMed
-
- Beckers NGM, Platteau P, Eijkemans MJ, Macklon NS, de Jong FH, Devroey P, Fauser BCJM. The early luteal phase administration of estrogen and progesterone does not induce premature luteolysis in normo-ovulatory women. European Journal of Endocrinology. 2006;155:355–363. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

