Mitochondrial DNA sequence variation is largely conserved at birth with rare de novo mutations in neonates
- PMID: 25687567
- PMCID: PMC4387098
- DOI: 10.1016/j.ajog.2015.02.009
Mitochondrial DNA sequence variation is largely conserved at birth with rare de novo mutations in neonates
Abstract
Objective: Mitochondrial DNA (mtDNA) encodes the proteins of the electron transfer chain to produce adenosine triphosphate through oxidative phosphorylation, and is essential to sustain life. mtDNA is unique from the nuclear genome in so much as it is solely maternally inherited (non-mendelian patterning), and shows a relatively high rate of mutation due to the absence of error checking capacity. While it is generally assumed that most new mutations accumulate through the process of heteroplasmy, it is unknown whether mutations initiated in the mother are inherited, occur in utero, or occur and accumulate early in life. The purpose of this study is to examine the maternally heritable and de novo mutation rate in the fetal mtDNA through high-fidelity sequencing from a large population-based cohort.
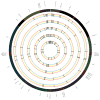
Study design: Samples were obtained from 90 matched maternal (blood) and fetal (placental) pairs. In addition, a smaller cohort (n = 5) of maternal (blood), fetal (placental), and neonatal (cord blood) trios were subjected to DNA extraction and shotgun sequencing. The whole genome was sequenced on the Illumina HiSeq platform (Illumina Inc., San Diego, CA), and haplogroups and mtDNA variants were identified through mapping to reference mitochondrial genomes (NC_012920).
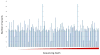
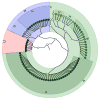
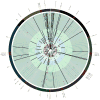
Results: We observed 665 single nucleotide polymorphisms and 82 insertions-deletions variants identified in the cohort at large. We achieved high sequencing depth of the mtDNA to an average depth of 65X (range, 20-171X) coverage. The proportions of haplogroups identified in the cohort are consistent with the patient's self-identified ethnicity (>90% Hispanic), and all maternal-fetal pairs mapped to the identical haplogroup. Only variants from samples with average depth >20X and allele frequency >1% were included for further analysis. While the majority of the maternal-fetal pairs (>90%) demonstrated identical variants at the single nucleotide level, we observed rare mitochondrial single nucleotide polymorphism discordance between maternal and fetal mitochondrial genomes.
Conclusion: In this first in-depth sequencing analysis of mtDNA from maternal-fetal pairs at the time of birth, a low rate of de novo mutations appears in the fetal mitochondrial genome. This implies that these mutations likely arise from the maternal heteroplasmic pool (eg, in the oocyte), and accumulate later in the offspring's life. These findings have key implications for both the occurrence and screening for mitochondrial disorders.
Keywords: maternal mitochondrial transmission; mitochondrial DNA mutations; mitochondrial DNA variation; mitochondrial DNA heritability; mitochondrial heteroplasmy.
Copyright © 2015 Elsevier Inc. All rights reserved.
Conflict of interest statement
Figures
References
-
- Lane N, Marin W. The energetics of genome complexity. Nature. 2010;467:929–934. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

