Structural basis for PPARγ transactivation by endocrine-disrupting organotin compounds
- PMID: 25687586
- PMCID: PMC4330522
- DOI: 10.1038/srep08520
Structural basis for PPARγ transactivation by endocrine-disrupting organotin compounds
Abstract
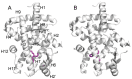
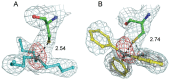
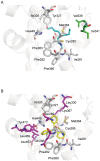
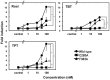
Organotin compounds such as triphenyltin (TPT) and tributyltin (TBT) act as endocrine disruptors through the peroxisome proliferator-activated receptor γ (PPARγ) signaling pathway. We recently found that TPT is a particularly strong agonist of PPARγ. To elucidate the mechanism underlying organotin-dependent PPARγ activation, we here analyzed the interactions of PPARγ ligand-binding domain (LBD) with TPT and TBT by using X-ray crystallography and mass spectroscopy in conjunction with cell-based activity assays. Crystal structures of PPARγ-LBD/TBT and PPARγ-LBD/TPT complexes were determined at 1.95 Å and 1.89 Å, respectively. Specific binding of organotins is achieved through non-covalent ionic interactions between the sulfur atom of Cys285 and the tin atom. Comparisons of the determined structures suggest that the strong activity of TPT arises through interactions with helix 12 of LBD primarily via π-π interactions. Our findings elucidate the structural basis of PPARγ activation by TPT.
Figures

References
-
- Fournier T. et al. The role of PPAR-gamma/RXR-alpha heterodimers in the regulation of human trophoblast invasion. Ann. N. Y. Acad. Sci. 973, 26–30 (2002). - PubMed
-
- Schaiff W. T. et al. Peroxisome proliferator-activated receptor-gamma modulates differentiation of human trophoblast in a ligand-specific manner. J. Clin. Endocrinol. Metab. 85, 3874–3881 (2000). - PubMed
-
- Tarrade A. et al. PPARgamma/RXRalpha heterodimers control human trophoblast invasion. J. Clin. Endocrinol. Metab. 86, 5017–5024 (2001). - PubMed
-
- Boyer I. J. Toxicity of dibutyltin, tributyltin and other organotin compounds to humans and to experimental animals. Toxicology 55, 253–298 (1989). - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

