Neuroinflammation and Aβ accumulation linked to systemic inflammation are decreased by genetic PKR down-regulation
- PMID: 25687824
- PMCID: PMC4330547
- DOI: 10.1038/srep08489
Neuroinflammation and Aβ accumulation linked to systemic inflammation are decreased by genetic PKR down-regulation
Abstract
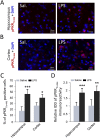
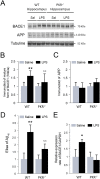
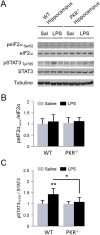
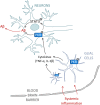
Alzheimer's disease (AD) is a neurodegenerative disorder, marked by senile plaques composed of amyloid-β (Aβ) peptide, neurofibrillary tangles, neuronal loss and neuroinflammation. Previous works have suggested that systemic inflammation could contribute to neuroinflammation and enhanced Aβ cerebral concentrations. The molecular pathways leading to these events are not fully understood. PKR is a pro-apoptotic kinase that can trigger inflammation and accumulates in the brain and cerebrospinal fluid of AD patients. The goal of the present study was to assess if LPS-induced neuroinflammation and Aβ production could be altered by genetic PKR down regulation. The results show that, in the hippocampus of LPS-injected wild type mice, neuroinflammation, cytokine release and Aβ production are significantly increased and not in LPS-treated PKR knock-out mice. In addition BACE1 and activated STAT3 levels, a putative transcriptional regulator of BACE1, were not found increased in the brain of PKR knock-out mice as observed in wild type mice. Using PET imaging, the decrease of hippocampal metabolism induced by systemic LPS was not observed in LPS-treated PKR knock-out mice. Altogether, these findings demonstrate that PKR plays a major role in brain changes induced by LPS and could be a valid target to modulate neuroinflammation and Aβ production.
Conflict of interest statement
The authors declare no competing financial interests.
Figures


References
-
- Duyckaerts C., Delatour B. & Potier M. C. Classification and basic pathology of Alzheimer disease. Acta Neuropathol 118, 5–36 (2009). - PubMed
-
- Hardy J. & Selkoe D. J. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science 297, 353–356 (2002). - PubMed
-
- McGeer E. G. & McGeer P. L. Neuroinflammation in Alzheimer's disease and mild cognitive impairment: a field in its infancy. J Alzheimers Dis 19, 355–361 (2010). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

