A pharmacokinetic binding model for bevacizumab and VEGF165 in colorectal cancer patients
- PMID: 25687989
- PMCID: PMC4365273
- DOI: 10.1007/s00280-015-2701-3
A pharmacokinetic binding model for bevacizumab and VEGF165 in colorectal cancer patients
Abstract
Purpose: To characterize the population pharmacokinetics of bevacizumab, its binding properties to VEGF165 and the effect of demographic data and VEGF-A polymorphisms on the interplay between bevacizumab serum pharmacokinetics and VEGF165 serum concentrations in patients with colorectal cancer stage IV.
Methods: Bevacizumab and VEGF165 data were collected from 19 adult patients with metastatic colorectal cancer enrolled in an observational clinical study. Bevacizumab was administered with one of the following combinations: 5-FU/Leucovorin/Irinotecan, 5-FU/Leucovorin/Oxaliplatin, Capecitabine/Irinotecan at doses ranging from 5 to 10 mg/kg every 2 or 3 weeks. Data analysis was performed using nonlinear mixed-effects modeling implemented in NONMEM 7.3.
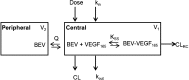
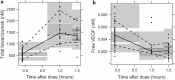
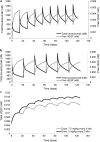
Results: A target-mediated drug disposition model adequately described bevacizumab concentration changes over time and its binding characteristics to VEGF165. The estimated clearance of bevacizumab was 0.18 L/day, the free VEGF165 levels at baseline were 212 ng/L, and the elimination rate constant of free VEGF165 was 0.401 day(-1). Body weight was allometrically included in all PK parameters.
Conclusion: The final model adequately described the pre- and post-dose concentrations of total bevacizumab and free VEGF165 in patients with colorectal cancer. Model parameters were consistent with those previously reported for patients with solid tumors. Correlations between the binding affinity of bevacizumab and the VEGF-2578C/A and VEGF-634G/C polymorphisms were noticed.
Figures
Similar articles
-
Lower exposure and faster clearance of bevacizumab in gastric cancer and the impact of patient variables: analysis of individual data from AVAGAST phase III trial.AAPS J. 2014 Sep;16(5):1056-63. doi: 10.1208/s12248-014-9631-6. Epub 2014 Jun 19. AAPS J. 2014. PMID: 24942210 Free PMC article. Clinical Trial.
-
Characterization of the long-term pharmacokinetics of bevacizumab following last dose in patients with resected stage II and III carcinoma of the colon.Cancer Chemother Pharmacol. 2013 Mar;71(3):575-80. doi: 10.1007/s00280-012-2031-7. Epub 2012 Dec 11. Cancer Chemother Pharmacol. 2013. PMID: 23228985
-
Population pharmacokinetic analysis of free and bound aflibercept in patients with advanced solid tumors.Cancer Chemother Pharmacol. 2013 Jul;72(1):167-80. doi: 10.1007/s00280-013-2182-1. Epub 2013 May 15. Cancer Chemother Pharmacol. 2013. PMID: 23673444 Clinical Trial.
-
Is there a benefit from addiction to anti-VEGF therapy in patients with colorectal cancer?Anticancer Res. 2013 Jun;33(6):2377-80. Anticancer Res. 2013. PMID: 23749885 Review.
-
Integration of novel agents in the treatment of colorectal cancer.Cancer Chemother Pharmacol. 2004 Sep;54 Suppl 1:S32-9. doi: 10.1007/s00280-004-0884-0. Cancer Chemother Pharmacol. 2004. PMID: 15309512 Review.
Cited by
-
Impact of time-varying cumulative bevacizumab exposures on survival: re-analysis of data from randomized clinical trial in patients with metastatic colo-rectal cancer.BMC Med Res Methodol. 2021 Jan 9;21(1):14. doi: 10.1186/s12874-020-01202-9. BMC Med Res Methodol. 2021. PMID: 33422006 Free PMC article. Clinical Trial.
-
Metronomic Temozolomide (mTMZ) and Bevacizumab-The Safe and Effective Frontier for Treating Metastatic Neuroendocrine Tumors (NETs): A Single-Center Experience.Cancers (Basel). 2023 Dec 1;15(23):5688. doi: 10.3390/cancers15235688. Cancers (Basel). 2023. PMID: 38067391 Free PMC article.
-
Phase II trial of docetaxel, bevacizumab, lenalidomide and prednisone in patients with metastatic castration-resistant prostate cancer.BJU Int. 2016 Oct;118(4):590-7. doi: 10.1111/bju.13412. Epub 2016 Feb 19. BJU Int. 2016. PMID: 26780387 Free PMC article. Clinical Trial.
-
Translation of Monoclonal Antibodies Pharmacokinetics from Animal to Human Using Physiologically Based Modeling in Open Systems Pharmacology (OSP) Suite: A Retrospective Analysis of Bevacizumab.Pharmaceutics. 2023 Aug 14;15(8):2129. doi: 10.3390/pharmaceutics15082129. Pharmaceutics. 2023. PMID: 37631343 Free PMC article.
-
Bringing Model-Based Prediction to Oncology Clinical Practice: A Review of Pharmacometrics Principles and Applications.Oncologist. 2016 Feb;21(2):220-32. doi: 10.1634/theoncologist.2015-0322. Epub 2015 Dec 14. Oncologist. 2016. PMID: 26668254 Free PMC article. Review.
References
-
- Avastin-H/C/000582-II/65 (2014) European Public Assessment Report. The European Medicines Agency. http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medici.... Accessed 20 Aug 2014
-
- Final Labeling Text, BLA 125085 (2013) Center for Drug Evaluation and Research. U.S. Food and Drug Administration. http://www.accessdata.fda.gov/drugsatfda_docs/label/2013/125085s285lbl.pdf. Accessed 20 Aug 2014
-
- Avastin® (2014) Genentech USA, Inc. http://www.avastin.com/patient?cid=gne_WE_00000083. Accessed 20 Aug 2014
-
- Parikh SS, Mehta HH, Desai BI. Advances in development of bevacizumab, a humanized antiangiogenic therapeutic monoclonal antibody targeting VEGF in cancer cells. Int J Pharm Biomed Sci. 2012;3:155–163.
-
- Tabrizi M, Roskos LK. Exposure–response relationships for therapeutic biologic products. In: Meibohm B, editor. Pharmacokinetics and pharmacodynamics of biotech drugs. Principles and case studies in drug development. Weinheim: WILEY-VCH Verlag GmbH & Co. KGaA; 2006. pp. 295–327.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

