Phosphorylation of targeting protein for Xenopus kinesin-like protein 2 (TPX2) at threonine 72 in spindle assembly
- PMID: 25688093
- PMCID: PMC4423697
- DOI: 10.1074/jbc.M114.591545
Phosphorylation of targeting protein for Xenopus kinesin-like protein 2 (TPX2) at threonine 72 in spindle assembly
Abstract
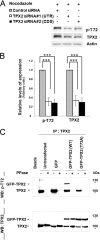
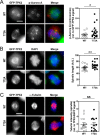
The human ortholog of the targeting protein for Xenopus kinesin-like protein 2 (TPX2) is a cytoskeletal protein that plays a major role in spindle assembly and is required for mitosis. During spindle morphogenesis, TPX2 cooperates with Aurora A kinase and Eg5 kinesin to regulate microtubule organization. TPX2 displays over 40 putative phosphorylation sites identified from various high-throughput proteomic screenings. In this study, we characterize the phosphorylation of threonine 72 (Thr(72)) in human TPX2, a residue highly conserved across species. We find that Cdk1/2 phosphorylate TPX2 in vitro and in vivo. Using homemade antibodies specific for TPX2 phosphorylated at Thr(72), we show that this phosphorylation is cell cycle-dependent and peaks at M phase. Endogenous TPX2 phosphorylated at Thr(72) does not associate with the mitotic spindle. Furthermore, ectopic GFP-TPX2 T72A preferentially concentrates on the spindle, whereas GFP-TPX2 WT distributes to both spindle and cytosol. The T72A mutant also increases the proportion of cells with multipolar spindles phenotype. This effect is associated with increased Aurora A activity and abnormally elongated spindles, indicative of higher Eg5 activity. In summary, we propose that phosphorylation of Thr(72) regulates TPX2 localization and impacts spindle assembly via Aurora A and Eg5.
Keywords: Antibody; Cell Cycle; Microtubule-associated Protein (MAP); Mitotic Spindle; Protein Phosphorylation.
© 2015 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures





Similar articles
-
Spindle pole regulation by a discrete Eg5-interacting domain in TPX2.Curr Biol. 2008 Apr 8;18(7):519-25. doi: 10.1016/j.cub.2008.02.077. Epub 2008 Mar 27. Curr Biol. 2008. PMID: 18372177 Free PMC article.
-
Characterization of the TPX2 domains involved in microtubule nucleation and spindle assembly in Xenopus egg extracts.Mol Biol Cell. 2004 Dec;15(12):5318-28. doi: 10.1091/mbc.e04-05-0385. Epub 2004 Sep 22. Mol Biol Cell. 2004. PMID: 15385625 Free PMC article.
-
A novel mechanism for activation of the protein kinase Aurora A.Curr Biol. 2003 Apr 15;13(8):691-7. doi: 10.1016/s0960-9822(03)00166-0. Curr Biol. 2003. PMID: 12699628
-
Regulation of Aurora-A kinase on the mitotic spindle.Chromosoma. 2003 Dec;112(4):159-63. doi: 10.1007/s00412-003-0265-1. Epub 2003 Nov 21. Chromosoma. 2003. PMID: 14634755 Review.
-
TPX2: of spindle assembly, DNA damage response, and cancer.Cell Mol Life Sci. 2014 Aug;71(16):3027-47. doi: 10.1007/s00018-014-1582-7. Epub 2014 Feb 21. Cell Mol Life Sci. 2014. PMID: 24556998 Free PMC article. Review.
Cited by
-
Identification of early stage recurrence endometrial cancer biomarkers using bioinformatics tools.Oncol Rep. 2020 Sep;44(3):873-886. doi: 10.3892/or.2020.7648. Epub 2020 Jun 16. Oncol Rep. 2020. PMID: 32705231 Free PMC article.
-
TPXL-1 activates Aurora A to clear contractile ring components from the polar cortex during cytokinesis.J Cell Biol. 2018 Mar 5;217(3):837-848. doi: 10.1083/jcb.201706021. Epub 2018 Jan 8. J Cell Biol. 2018. PMID: 29311228 Free PMC article.
-
Aurora-A Kinase as a Promising Therapeutic Target in Cancer.Front Oncol. 2016 Jan 6;5:295. doi: 10.3389/fonc.2015.00295. eCollection 2015. Front Oncol. 2016. PMID: 26779440 Free PMC article. Review.
-
Cross-Talk between AURKA and Plk1 in Mitotic Entry and Spindle Assembly.Front Oncol. 2015 Dec 23;5:283. doi: 10.3389/fonc.2015.00283. eCollection 2015. Front Oncol. 2015. PMID: 26779436 Free PMC article. Review.
-
Non-centrosomal TPX2-Dependent Regulation of the Aurora A Kinase: Functional Implications for Healthy and Pathological Cell Division.Front Oncol. 2016 Apr 15;6:88. doi: 10.3389/fonc.2016.00088. eCollection 2016. Front Oncol. 2016. PMID: 27148480 Free PMC article. Review.
References
-
- Heidebrecht H. J., Buck F., Steinmann J., Sprenger R., Wacker H. H., Parwaresch R. (1997) p100: a novel proliferation-associated nuclear protein specifically restricted to cell cycle phases S, G2, and M. Blood 90, 226–233 - PubMed
-
- Gruss O. J., Wittmann M., Yokoyama H., Pepperkok R., Kufer T., Silljé H., Karsenti E., Mattaj I. W., Vernos I. (2002) Chromosome-induced microtubule assembly mediated by TPX2 is required for spindle formation in HeLa cells. Nat. Cell Biol. 4, 871–879 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

