The expanding role of mTOR in cancer cell growth and proliferation
- PMID: 25688110
- PMCID: PMC5943824
- DOI: 10.1093/mutage/geu045
The expanding role of mTOR in cancer cell growth and proliferation
Abstract
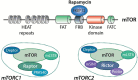
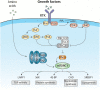
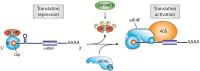
The mechanistic/mammalian target of rapamycin (mTOR) is a conserved protein kinase that controls several anabolic processes required for cell growth and proliferation. As such, mTOR has been implicated in an increasing number of pathological conditions, including cancer, obesity, type 2 diabetes and neurodegeneration. As part of the mTOR complex 1 (mTORC1), mTOR regulates cell growth by promoting the biosynthesis of proteins, lipids and nucleic acids. Several mTORC1 substrates have been shown to regulate protein synthesis, including the eukaryotic initiation factor 4E (eIF4E)-binding proteins (4E-BPs) and the ribosomal S6 kinases (S6Ks) 1 and 2. In this work, we focus on the signalling pathways that lie both upstream and downstream of mTORC1, as well as their relevance to human pathologies. We further discuss pharmacological approaches that target mTOR and their applications for the treatment of cancer.
© The Author 2015. Published by Oxford University Press on behalf of the UK Environmental Mutagen Society. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures

Similar articles
-
Increased milk protein synthesis in response to exogenous growth hormone is associated with changes in mechanistic (mammalian) target of rapamycin (mTOR)C1-dependent and independent cell signaling.J Dairy Sci. 2013 Apr;96(4):2327-2338. doi: 10.3168/jds.2012-6267. Epub 2013 Feb 22. J Dairy Sci. 2013. PMID: 23462168
-
Ubiquilin-mediated Small Molecule Inhibition of Mammalian Target of Rapamycin Complex 1 (mTORC1) Signaling.J Biol Chem. 2016 Mar 4;291(10):5221-33. doi: 10.1074/jbc.M115.691584. Epub 2016 Jan 6. J Biol Chem. 2016. PMID: 26740621 Free PMC article.
-
Distinct signaling mechanisms of mTORC1 and mTORC2 in glioblastoma multiforme: a tale of two complexes.Adv Biol Regul. 2015 Jan;57:64-74. doi: 10.1016/j.jbior.2014.09.004. Epub 2014 Sep 18. Adv Biol Regul. 2015. PMID: 25442674
-
mTOR-dependent cell survival mechanisms.Cold Spring Harb Perspect Biol. 2012 Dec 1;4(12):a008771. doi: 10.1101/cshperspect.a008771. Cold Spring Harb Perspect Biol. 2012. PMID: 23124837 Free PMC article. Review.
-
A growing role for mTOR in promoting anabolic metabolism.Biochem Soc Trans. 2013 Aug;41(4):906-12. doi: 10.1042/BST20130041. Biochem Soc Trans. 2013. PMID: 23863154 Review.
Cited by
-
The Role of Epac in Cancer Progression.Int J Mol Sci. 2020 Sep 5;21(18):6489. doi: 10.3390/ijms21186489. Int J Mol Sci. 2020. PMID: 32899451 Free PMC article. Review.
-
HSF1: Guardian of Proteostasis in Cancer.Trends Cell Biol. 2016 Jan;26(1):17-28. doi: 10.1016/j.tcb.2015.10.011. Epub 2015 Nov 18. Trends Cell Biol. 2016. PMID: 26597576 Free PMC article. Review.
-
Discovery of a nitroaromatic nannocystin with potent in vivo anticancer activity against colorectal cancer by targeting AKT1.Acta Pharmacol Sin. 2024 May;45(5):1044-1059. doi: 10.1038/s41401-024-01231-w. Epub 2024 Feb 7. Acta Pharmacol Sin. 2024. PMID: 38326625 Free PMC article.
-
EGFR/c-Met and mTOR signaling are predictors of survival in non-small cell lung cancer.Ther Adv Med Oncol. 2020 Sep 14;12:1758835920953731. doi: 10.1177/1758835920953731. eCollection 2020. Ther Adv Med Oncol. 2020. PMID: 32973931 Free PMC article.
-
Inactivation of the LKB1-AMPK signaling pathway does not contribute to salivary gland tumor development - a short report.Cell Oncol (Dordr). 2016 Aug;39(4):389-96. doi: 10.1007/s13402-016-0290-8. Epub 2016 Aug 1. Cell Oncol (Dordr). 2016. PMID: 27480082
References
-
- Yang Q., Guan K. L. (2007)Expanding mTOR signaling. Cell Res., 17, 666–681. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

